Abstract
Introduction
Cardiovascular molecular imaging is a rapidly evolving field of research, aiming to image and quantify molecular and cellular targets in vivo. MR imaging has some inherent properties that make it very suitable for cardiovascular molecular imaging. Until now, only a limited number of studies have been published on cardiovascular molecular imaging using MR imaging.
Review
In the current review, MR techniques that have already shown potential are discussed. Metabolic MR imaging can be performed by 31P-MR spectroscopy, 23Na MR spectroscopy and 1H-MR spectroscopy; some examples are shown. Furthermore, a concise overview is given of several aspecific and specific contrast agents for cardiovascular molecular MR imaging, such as gadolinium-based contrast agents, iron oxide MR contrast agents and fibrin-targeted MR contrast agents.
Conclusion
We expect that in the next decade currently promising MR molecular imaging agents will be introduced into the clinical arena to guide diagnosis and therapy of cardiovascular disease.
Similar content being viewed by others
Introduction
Conventional imaging modalities, including magnetic resonance (MR), are primarily based on anatomical, functional or metabolic properties to study (patho)physiology. Molecular imaging is a rapidly evolving field of research, aiming to image and quantify molecular and cellular targets in vivo. Molecular imaging can be applied to a wide range of scientific and clinical fields of interest. One of the most promising applications of molecular imaging is in the field of cardiovascular imaging. Imaging of cardiac anatomy, dimensions and function has some limitations concerning, for example, prediction of therapy outcome. Addition of specific information on, for instance, plaque composition and total plaque burden can be very helpful in guiding therapy.
Imaging of molecular processes is desirable because cardiovascular disease may be detected earlier, risk stratification may be more accurate, monitoring of innovative therapies may be improved, or a more accurate prognosis may be provided [1].
MR imaging has some inherent properties that make it very suitable for cardiovascular molecular imaging. The interaction between inherent tissue properties and specific contrast agents may lead to more specific clinical conclusions and prediction of therapy outcome. Thereby, cardiovascular molecular MR imaging may help in diagnosing cardiovascular disease, and in deciding whether expected beneficial effects of (invasive) therapy counterbalance the risk of complications of therapy.
A conventional approach to molecular MR imaging concerns MR spectroscopy. Furthermore, there are two main innovative contrast agents that may be used clinically soon: (1) iron oxide MR contrast agents and (2) fibrin-targeted MR contrast agents.
MR spectroscopy
MR spectroscopy (MRS) allows non-invasive characterisation of myocardial metabolism. In principle, MRS is a pure form of molecular ‘imaging’ technique. Clinically, several nuclei allow noninvasive MRS of the heart. Initially, human MRS research was focussed on the 31P nucleus to study high-energy phosphate metabolism. An example concerning the effects of diabetes type 2 on myocardial high-energy phosphate metabolism is shown in Fig. 1 [2]. Another interesting new application of 31P-MRS was published by Smith et al. [3], who measured myocardial creatine kinase (CK) metabolite concentrations and adenosine triphosphate synthesis through CK, the primary energy reserve of the heart, to test the hypothesis that ATP flux through CK is impaired in patients with left ventricular hypertrophy (LVH) and chronic heart failure. It turned out that myocardial ATP levels were normal, but creatine phosphate levels were 35% lower in LVH patients than in normal subjects. Furthermore, the myocardial CK rate constant was normal in LVH, but halved in patients with LVH combined with chronic heart failure. Thereby, they could show that it is not the relative or absolute CK metabolite pool size but rather the kinetics of ATP turnover through CK that distinguishes failing from non-failing hypertrophic hearts. These observations support the hypothesis that a deficit in myofibrillar energy delivery contributes to chronic heart failure pathophysiology in human LVH. The same technique was applied by Weiss et al. to study ATP flux through CK in the normal, stressed and failing human heart [4]. The latter studies are elegant examples of the capability of MR to measure non-invasively the concentration of metabolites and even the rate constant of enzyme turnover.
Left panel: planscan of the volume of interest on transverse and sagittal spin-echo MR images. Right panel: phosphorus-31 magnetic resonance spectroscopy (31P-MRS) obtained at rest from the anterior left ventricular wall in a patient with diabetes type 2 (left) and a healthy subject (right). Note the decreased signal amplitude of phosphocreatine in the type 2 diabetes patient. Courtesy of Diamant et al. [2]
Another interesting application of metabolic imaging is 23Na MR imaging, as show by Jansen et al. They applied this innovative spectroscopic imaging technique as a diagnostic modality for early detection of myocardial ischaemia and viability in a rat model [5]. They tested whether 23Na MR imaging can be used to assess viability after low-flow ischaemia. 23Na MR chemical shift imaging was alternated with 23Na MR spectroscopy. Na image intensity increased with increasing severity of ischaemia. 23Na image intensity at end low-flow ischaemia was well correlated with CK release during reperfusion, as well as with infarct size. Therefore, their study indicates that 23Na MR imaging is a promising tool for the assessment of myocardial viability. Ouwerkerk et al. applied 23Na MR imaging to measure cardiac tissue sodium concentrations in the human myocardium [6]. They used a surface coil at 1.5 T MR to non-invasively quantify regional myocardial sodium concentrations in the left ventricular free wall, septum and adipose tissue. Their 23Na MR imaging results were consistent with prior invasive measurements on biopsy and autopsy specimens.
In the past, 1H-MRS of the myocardium was first applied to non-invasively study creatine depletion in non-viable infarcted myocardium [7]. Total creatine was measured in the posterior and anterior left ventricle and septum, and was significantly lower in regions of infarction than in non-infarcted regions of myocardium in patients or in the myocardium of healthy controls. Therefore, they showed for the first time that spatially localised 1H-MRS can be used to measure total creatine non-invasively throughout the human heart. The detection of regional creatine depletion may provide a metabolic means to distinguish healthy from infarcted non-viable myocardium. Szczepaniak et al. used 1H-MRS to measure myocardial lipid content [8]. Studies in rat tissue ex vivo and in healthy humans in vivo provided evidence that 1H-MRS constitutes a reproducible technique for the measurement of myocardial triglyceride. Increased myocardial triglyceride content was accompanied by elevated LV mass and suppressed septal wall thickening as measured by cardiac imaging. More recently, 1H-MRS of the human myocardium was improved by implementing the respiratory navigator technique to monitor diaphragmatic motion, and thereby correct data acquisition prospectively for breathing motion. First (unpublished) results from our institution show improved reproducibility of human cardiac 1H-MRS measurements when using the respiratory navigator technique, as compared with conventional continuous breathing. Respiratory navigator gated 1H-MRS was recently applied in an experimental setting in our institution to evaluate the effects of a very low calorie diet on myocardial triglyceride content. First (unpublished) results show that after a short very low calorie diet, there is an increase in intramyocardial triglyceride content (Fig. 2).
The surface coil was positioned just below the mitral valve level of the heart (a, b). Spectroscopic volume localisation in the interventricular septum on four-chamber (c) and short axis (d) views. Special care was taken to avoid contamination from epicardial fat. (e) Typical water-suppressed 1H-MR spectrum of myocardial tissue located in the interventricular septum. Peak heights are in arbitrary units
Gadolinium-based contrast agents
Gadolinium-based contrast agents can be applied to study regional myocardial perfusion. After a rapid intravenous contrast injection, there is marked signal enhancement first in the RV cavity, then in the LV cavity, and subsequently in the LV myocardium [9]. The peak signal intensity is related to the concentration of the contrast agent in the local tissue and is directly proportional to the coronary blood flow. Perfusion MR at rest and after infusion of pharmacological agents (adenosine and persantine) have been compared with standard methods (angiography or radionuclide scintigraphy) and demonstrated reasonable sensitivity (67–83%) and specificity (75–100%) [9].
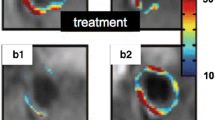
Currently, multiple MR imaging techniques are available to assess myocardial viability. Cardiovascular MR imaging can be used to assess end-diastolic wall thickness and contractile function at rest [10]. Segments with an end-diastolic wall thickness <6 mm most likely represent transmural scar formation, and contractile function will not improve after myocardial revascularisation. Dobutamine MR imaging can be used to evaluate contractile reserve, in a similar manner to dobutamine echocardiography. Gadolinium contrast-enhanced MR imaging [11] allows for detection of the extent and transmurality of scar tissue (Fig. 3). Recently reported sensitivity and specificity are in the range of 74% and 82% respectively.
Left panel: short-axis MR images at rest and during dobutamine stress. Note the lack of improvement in myocardial wall motion in the anteroseptal region when dobutamine stress is applied. Right panel: delayed gadolinium contrast-enhanced MR images in two-chamber, four-chamber and short-axis views. Note the almost transmural delayed enhancement of the anteroseptal/apical region, corresponding to the region without contractile response due to dobutamine stress in the left panel. The anteroseptal region is considered as ‘non-viable’ myocardial tissue
Based on as yet unpublished scientific developments, it is expected that gadolinium-based delayed enhancement of the vessel wall may become reality. This MR imaging technique may allow fast total body screening for total plaque burden, an important predictor for morbidity and mortality.
In general, gadolinium-based contrast agents are not perfectly suited for molecular imaging because of the inherent high threshold of detectability. Therefore, new contrast agents are under development to potentiate the effect of distortion of the magnetic field.
Iron oxide MR contrast agents
Superparamagnetic iron oxide (SPIO) particles can be detected at micromolar concentrations of iron, and offer sufficient sensitivity for MR imaging [12]. SPIO-based cellular imaging has become an established technique to label and detect cells of interest. Imaging of macrophage activity was the initial and is still the most significant application, with several products either approved for or in clinical trials [12]. Another exciting application of SPIOs is labelling of myocardial stem cells. In a swine model for myocardial infarction, magnetically labelled stem cells were injected in the infarcted myocardial region (Fig. 4). Using delayed contrast-enhanced MR imaging, the infarcted area can be identified with high accuracy. New technical developments may even allow specific delivery of magnetically labelled therapeutics to the infarcted myocardial region [12]. Combined with measurements of myocardial function, MR imaging seems an excellent modality for planning, delivery and follow-up of myocardial stem cells as therapy for ischaemic heart disease.
Detection of delivery and migration of Feridex-labelled myocardial stem cells in a swine model. Hypointense lesions in spin-echo (SE),gradient-echo (GRE) and delayed-enhanced (Delayed) MR imaging (upper panel) of injection sites (arrows) within 24 h of intramyocardial injection. Cells were injected in the myocardial infarct (MI). Long-axis MR images (lower panel) show hypointense lesions (arrows) caused by labelled myocardial stem cells acquired within 24 h and 1 week. LV left ventricle, RV right ventricle. Courtesy of Bulte andKraitchman [12]
Another promising application of SPIO MR imaging is visualisation of vessel wall inflammation. SPIOs are ‘digested’ by macrophages, involved in inflammatory processes. Imaging of the SPIO-induced magnetic inhomogeneities allows for imaging of inflammation. Such an approach is currently only available in a research setting; it is, however, expected that these contrast agents will become available for clinical application soon.
Fibrin-targeted MR contrast agents
Exciting recent developments allow selective and non-invasive molecular MR imaging of thrombus [13]. The principle of the contrast agent is that it is targeted to fibrin. In an elegant study by Spuentrup et al., a swine model was used to test the innovative fibrin-targeted MR imaging contrast agent, which can be administered intravenously [14]. The imaging protocol consisted of coronary MR angiography to demonstrate the lumen of a coronary artery, combined with molecular thrombus MR imaging. Thereby, anatomical information could be linked to specific information of vessel wall components. In an area of focal coronary artery stenosis, intraluminal thrombus could be detected (Fig. 5).
Coronary thrombus visualisation with a fibrin-targeted molecular MR imaging contrast agent. Left panel: double-oblique white blood coronary MR angiography (multiplanar reconstruction) demonstrating the lumen of the left anterior descending artery with bright signal (arrowheads). Right panel: double-oblique MR images after administration of the fibrin-targeted contrast agent (multiplanar reconstruction, same orientation). Note the increased signal (arrow) in the left anterior descending coronary artery, corresponding to thrombus. Courtesy of Spuentrup et al. [14]
The same contrast agent can be applied to detect, for example, right atrial thrombus, a potential source of more distal emboli (Fig. 6). An atrial clot could be visualised easily with this molecular MR imaging technique, by intravenous administration of the fibrin-targeted contrast agent. Furthermore, MR clot imaging can be combined with functional imaging of the heart in the same imaging session. An even more exciting application of this fibrin-targeted contrast agent is detection of pulmonary emboli (Fig. 7).
Molecular MR imaging of atrial clot in a swine model. The left panel shows pre-contrast (upper) and post-contrast (lower) coronal images. Note the presence of high MR signal in the area of the right atrium, indicating an atrial clot. The right panel shows increased MR signal in the left atrium (LA), corresponding to a left atrial clot (arrow). These clots are potential sources for more distal emboli. LV left ventricle. Courtesy of Spuentrup et al. [14]
Examples of molecular MR imaging of pulmonary embolus. Two examples are shown, each consisting of three adjacent coronal slices (horizontal). The MR imaging technique is such that signal from surrounding blood pool and lung parenchyma is suppressed. The upper row shows pulmonary embolus (arrow) in the right lower lobe. The lower panel shows bilateral pulmonary emboli (arrows) after intravenous administration of fibrin-targeted MR imaging contrast agent. Courtesy of Spuentrup et al. [14]
The above-described applications of molecular MR imaging may be especially suitable for fast screening for cardiovascular disease in an emergency setting. Patients presenting with chest pain in the emergency room can be studied by MR imaging to confirm or rule out ischaemic heart disease or pulmonary embolus. Molecular MR imaging using fibrin-targeted contrast agents allows selective visualisation of acute coronary, cardiac and pulmonary thrombi. Additional functional cardiac imaging can help determine the functional effects of detected thrombi.
Conclusion
Molecular MR imaging is an exciting and rapidly evolving new area of cardiovascular imaging. MR imaging seems very suitable for molecular imaging, although many technical difficulties have to be overcome. The main current limitation is the low sensitivity of MR imaging to detect small changes in magnet homogeneity. We expect that in the next decade, currently promising MR molecular imaging agents will be introduced into the clinical arena to guide diagnosis and therapy of cardiovascular disease.
References
Jaffer FA, Weissleder R. Seeing within: molecular imaging of the cardiovascular system. Circ Res 2004;94 4:433–45.
Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol 2003;42 2:328–35.
Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 2006;114:1151–8.
Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. PNAS 2005;102;808–13.
Jansen MA, Van Emous JG, Nederhoff MGJ, Van Echteld CJA. Assessment of myocardial viability by intracellular 23Na magnetic resonance imaging. Circulation 2004;110:3457–64.
Ouwerkerk R, Weiss RG, Bottomley PA. Measuring human cardiac tissue sodium concentrations using surface coils, adiabatic excitation, and twisted projection imaging with minimal T2 losses. J Magn Reson Imaging 2005;21:546–55.
Bottomley PA, Weiss RG. Non-invasive magnetic-resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet 1998;351:714–8.
Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, et al. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med 2003;49:417–23.
Lima JAC, Desai MY. Cardiovascular magnetic resonance imaging: current and emerging applications. J Am Coll Cardiol 2004;44:1164–71.
Baer FM, Theissen P, Schneider CA, Voth E, Sechtem U, Schicha H, et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol 1998;31 5:1040–8.
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343 20:1445–53.
Bulte JW, Kraitchman D. Iron oxide MR contrast agents for molecular and cellular imaging. NMR in Biomedicine 2004;17:484–99.
Spuentrup E, Buecker A, Katoh M, Wiethoff AJ, Parsons EC Jr, Botnar RM, et al. Molecular magnetic resonance imaging of coronary thrombosis and pulmonary emboli with a novel fibrin-targeted contrast agent. Circulation 2005;111 11:1377–82.
Spuentrup E, Fausten B, Kinzel S, Wiethoff AJ, Botnar RM, Graham PB, et al. Molecular magnetic resonance imaging of atrial clots in a swine model. Circulation 2005;112 3:396–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lamb, H.J., van der Meer, R.W., de Roos, A. et al. Cardiovascular molecular MR imaging. Eur J Nucl Med Mol Imaging 34 (Suppl 1), 99–104 (2007). https://doi.org/10.1007/s00259-007-0444-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0444-z