Abstract
Purpose
It is generally assumed that the biodistribution and pharmacokinetics of radiolabelled antibodies remain similar between dosimetric and therapeutic injections in radioimmunotherapy. However, circulation half-lives of unlabelled rituximab have been reported to increase progressively after the weekly injections of standard therapy doses. The aim of this study was to evaluate the evolution of the pharmacokinetics of repeated 131I-rituximab injections during treatment with unlabelled rituximab in patients with non-Hodgkin’s lymphoma (NHL).
Methods
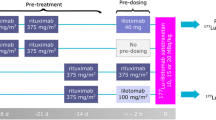
Patients received standard weekly therapy with rituximab (375 mg/m2) for 4 weeks and a fifth injection at 7 or 8 weeks. Each patient had three additional injections of 185 MBq 131I-rituximab in either treatment weeks 1, 3 and 7 (two patients) or weeks 2, 4 and 8 (two patients). The 12 radiolabelled antibody injections were followed by three whole-body (WB) scintigraphic studies during 1 week and blood sampling on the same occasions. Additional WB scans were performed after 2 and 4 weeks post 131I-rituximab injection prior to the second and third injections, respectively.
Results
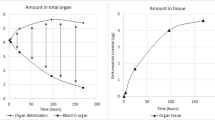
A single exponential radioactivity decrease for WB, liver, spleen, kidneys and heart was observed. Biodistribution and half-lives were patient specific, and without significant change after the second or third injection compared with the first one. Blood T1/2β, calculated from the sequential blood samples and fitted to a bi-exponential curve, was similar to the T1/2 of heart and liver but shorter than that of WB and kidneys. Effective radiation dose calculated from attenuation-corrected WB scans and blood using Mirdose3.1 was 0.53+0.05 mSv/MBq (range 0.48–0.59 mSv/MBq). Radiation dose was highest for spleen and kidneys, followed by heart and liver.
Conclusion
These results show that the biodistribution and tissue kinetics of 131I-rituximab, while specific to each patient, remained constant during unlabelled antibody therapy. RIT radiation doses can therefore be reliably extrapolated from a preceding dosimetry study.




Similar content being viewed by others
References
Wiseman GA, White CA, Stabin M, Dunn WL, Erwin W, Dahlbom M, et al. Phase I/II 90Y-Zevalin (yttrium-90 ibritumomab tiuxetan, IDEC-Y2B8) radioimmunotherapy dosimetry results in relapsed or refractory non-Hodgkin’s lymphoma. Eur J Nucl Med 2000;27:766–77.
Gordon LI, Molina A, Witzig T, Emmanouilides C, Raubtischek A, Darif M, et al. Durable responses after ibritumomab tiuxetan radioimmunotherapy for CD20+ B-cell lymphoma: long term follow-up of a phase I/II study. Blood 2004;103:4429–31.
Wahl RL, Zasadny KR, MacFarlane D, Francis IR, Ross CW, Estes J, et al. Iodine-131 anti-B1 antibody for B-cell lymphoma: an update on the Michigan phase I experience. J Nucl Med 1998;39:21S–7S.
Kaminski MS, Estes J, Zasadny KR, Francis IR, Ross CW, Tuck M, et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood 2000;96:1259–66.
Gopal AK, Gooley TA, Maloney DG, Petersdorf SH, Eary JF, Rajendran JG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood 2003;102:2351–7.
Juweid ME. Radioimmunotherapy of B-cell non-Hodgkin’s lymphoma: from clinical trials to clinical practice. J Nucl Med 2002;43:1507–29.
Goldenberg DM. The role of radiolabeled antibodies in the treatment of non-Hodgkin’s lymphoma: the coming of age of radioimmunotherapy. Crit Rev Oncol Hematol 2001;39:195–201.
Press OW, Rasey J. Principles of radioimmunotherapy for hematologists and oncologists. Semin Oncol 2000;27:62–73.
Behr TM, Griesinger F, Riggert J, Gratz S, Béhé M, Kaufmann CC, et al. High-dose myeloablative radioimmunotherapy of mantle cell non-Hodgkin lymphoma with the iodine-131-labeled chimeric anti-CD20 antibody C2B8 and autologous stem cell support. Results of a pilot study. Cancer 2002;94:1363–72.
Scheidhauer K, Wolf I, Baumgartl HJ, Von Schilling C, Schmidt B, Reidel G, et al. Biodistribution and kinetics of 131I-labelled anti-CD20 MAB IDEC-C2B8 (rituximab) in relapsed non-Hodgkin’s lymphoma. Eur J Nucl Med Mol Imaging 2002;29:1276–82.
Turner JH, Martindale AA, Boucek J, Claringbold PG, Leahy MF. 131I-Anti CD20 radioimmunotherapy of relapsed or refractory non-Hodgkin’s lymphoma: a phase II clinical trial of a nonmyeloablative dose regimen of chimeric rituximab radiolabeled in a hospital. Cancer Biother Radiopharm 2003;18:513–24.
McDevitt MR, Ma D, Lai LT, Simon J, Borchardt P, Frank RK, et al. Tumor therapy with targeted atomic nanogenerators. Science 2001;294:1537–40.
Beyer GJ, Miederer M, Vranjes-Duric S, Comor JJ, Kunzi G, Hartley O, et al. Targeted alpha therapy in vivo: direct evidence for single cancer cell kill using 149Tb-rituximab. Eur J Nucl Med Mol Imaging 2004;31:547–54.
Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, et al. Association of serum rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol 1998;9:995–1001.
Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin’s lymphoma. J Clin Oncol 1997;15:3266–74.
Schaffland AO, Buchegger F, Kosinski M, Antonescu C, Paschoud C, Grannavel C, et al. 131I-Rituximab: relationship of immunoreactivity with specific activity. J Nucl Med 2004;45:1784–90.
Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood 1994;83:435–45.
Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA Jr. Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods 1984;72:77–89.
Wahl RL, Kroll S, Zasadny KR. Patient-specific whole-body dosimetry: principles and a simplified method for clinical implementation. J Nucl Med 1998;39:14S–20S.
Stabin MG. MIRDOSE: personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 1996;37:538–46.
Sgouros G. Bone marrow dosimetry for radioimmunotherapy: theoretical considerations. J Nucl Med 1993;34:689–94.
Scatchard G. The attractions of proteins for small molecules and ions. Ann N Y Acad Sci 1949;51:660–72.
Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood 1997;90:2188–95.
Davies AJ, Rohatiner AZ, Howell S, Britton KE, Owens SE, Micallef IN, et al. Tositumomab and iodine I 131 tositumomab for recurrent indolent and transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol 2004;22:1469–79.
Divgi CR, O’Donoghue JA, Welt S, O’Neel J, Finn R, Motzer RJ, et al. Phase I clinical trial with fractionated radioimmunotherapy using 131I-labeled chimeric G250 in metastatic renal cancer. J Nucl Med 2004;45:1412–21.
Britton KE. Radioimmunotherapy of non-Hodgkin’s lymphoma. J Nucl Med 2004;45:924–5.
Eary JF, Krohn KA, Press OW, Durack L, Bernstein ID. Importance of pre-treatment radiation absorbed dose estimation for radioimmunotherapy of non-Hodgkin’s lymphoma. Nucl Med Biol 1997;24:635–8.
Acknowledgements
We thankfully acknowledge support from the Swiss Cancer League No KFS 991-02-2000 and excellent technical assistance from the staff of the Nuclear Medicine Department.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antonescu, C., Bischof Delaloye, A., Kosinski, M. et al. Repeated injections of 131I-rituximab show patient-specific stable biodistribution and tissue kinetics. Eur J Nucl Med Mol Imaging 32, 943–951 (2005). https://doi.org/10.1007/s00259-005-1798-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1798-8