Abstract
Purpose
This study reports on the whole-body biodistribution and radiation dosimetry of [11C]raclopride, a dopamine D2 receptor antagonist.
Methods
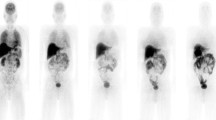
In three healthy male volunteers, whole-body scans were performed up to 2 h following i.v. injection of 320±65 MBq [11C]raclopride. Transmission scans (3 min per step, eight or nine steps according to the height of the subject) in 2D mode were used for subsequent attenuation correction of emission scans. Emission scans (1 min per step, eight or nine steps) were acquired over 2 h. Venous blood samples and urine were collected up to 2 h after injection of the radiotracer. For each subject, the percentage of injected activity measured in regions of interest over brain, intestine, lungs, kidneys and liver was fitted to a mono-exponential model, as an uptake phase followed by a mono-exponential washout, for urinary bladder to generate time–activity curves. Using the MIRD method, several source organs were considered in estimating residence time and mean effective radiation absorbed doses.
Results
Blood pressure and ECG findings remained unchanged after tracer injection. The analysed blood and urine pharmacological parameters did not change significantly after [11C]raclopride injection. The primary routes of clearance were renal and intestinal. Ten minutes after injection, high activities were observed in the gall-bladder, kidneys and liver. High activity was observed in the gall-bladder during the whole study. The kidneys, urinary bladder wall, liver and gall-bladder received the highest absorbed doses. The average effective dose of [11C]raclopride was estimated to be 6.7±0.4 μSv/MBq.
Conclusion
The amount of [11C]raclopride required for adequate dopamine D2 receptor imaging results in an acceptable effective dose equivalent, permitting two or three repeated clinical PET imaging studies, with the injection of 222 MBq for each study.


Similar content being viewed by others
References
Farde L, Ehrin E, Eriksson L, Greitz T, Hall H, Hedstrom CG, et al. Substituted benzamides as ligands for visualization of dopamine receptor binding in the human brain by positron emission tomography. Proc Natl Acad Sci U S A 1985;82:3863–7.
Farde L, Hall H, Ehrin E, Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science 1986;231:258–61.
Halldin C, Farde L, Hall H, Hogberg T, Printz G, Pulka W, et al. Synthesis of five benzamide analogs: comparison with positron emission tomography. Acta Radiol Suppl 1991;376:121–2.
Antonini A, Leenders KL. Dopamine D2 receptors in normal human brain: effect of age measured by positron emission tomography (PET) and [11C]-raclopride. Ann N Y Acad Sci 1993;695:81–5.
Rinne JO, Hietala J, Ruotsalainen U, Sako E, Laihinen A, Nagren K, et al. Decrease in human striatal dopamine D2 receptor density with age: a PET study with [11C]raclopride. J Cereb Blood Flow Metab 1993;13:310–4.
Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR, et al. Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res 1996;67:11–6.
Wang GJ, Volkow ND, Fowler JS, Logan J, Gur R, Netusil N, et al. Age associated decrements in dopamine D2 receptors in thalamus and in temporal insula of human subjects. Life Sci 1996;59:PL31–5.
Brooks DJ, Ibanez V, Sawle GV, Playford ED, Quinn N, Mathias CJ, et al. Striatal D2 receptor status in patients with Parkinson’s disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11C-raclopride and positron emission tomography. Ann Neurol 1992;31:184–92.
Antonini A, Vontobel P, Psylla M, Gunther I, Maguire PR, Missimer J, et al. Complementary positron emission tomographic studies of the striatal dopaminergic system in Parkinson’s disease. Arch Neurol 1995;52:1183–90.
Antonini A, Leenders KL, Eidelberg D. [11C]raclopride-PET studies of the Huntington’s disease rate of progression: relevance of the trinucleotide repeat length. Ann Neurol 1998;43:253–5.
Hilker R, Klein C, Ghaemi M, Kis B, Strotmann T, Ozelius LJ, et al. Positron emission tomographic analysis of the nigrostriatal dopaminergic system in familial Parkinsonism associated with mutations in the Parkin gene. Ann Neurol 2001;49:367–76.
Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL. Long-term changes of striatal dopamine D2 receptors in patients with Parkinson’s disease: a study with positron emission tomography and [11C]raclopride. Mov Disord 1997;12:33–8.
Rinne JO, Laihinen A, Rinne UK, Nagren K, Bergman J, Ruotsalainen U. PET study on striatal dopamine D2 receptor changes during the progression of early Parkinson’s disease. Mov Disord 1993;8:134–8.
Ginovart N, Lundin A, Farde L, Halldin C, Backman L, Swahn CG, et al. PET study of the pre- and post-synaptic dopaminergic markers for the neurodegenerative process in Huntington’s disease. Brain 1997;120:503–14.
Bench CJ, Lammertsma AA, Dolan RJ, Grasby PM, Warrington SJ, Gunn K, et al. Dose dependent occupancy of central dopamine D2 receptors by the novel neuroleptic CP-88,059-01: a study using positron emission tomography and 11C-raclopride. Psychopharmacology (Berl) 1993;112:308–14.
Nyberg S, Farde L, Halldin C. A PET study of 5-HT2 and D2 dopamine receptor occupancy induced by olanzapine in healthy subjects. Neuropsychopharmacology 1997;16:1–7.
Hagberg G, Gefvert O, Bergstrom M, Wieselgren IM, Lindstrom L, Wiesel FA, et al. N-[11C]methylspiperone PET, in contrast to [11C]raclopride, fails to detect D2 receptor occupancy by an atypical neuroleptic. Psychiatry Res 1998;82:147–60.
Fowler JS, Wang GJ, Volkow ND, Ieni J, Logan J, Pappas N, et al. PET Studies of the effect of the antidepressant drugs nefazodone or paroxetine on [11C]raclopride binding in human brain. Clin Positron Imaging 1999;2:205–9.
Yokoi F, Grunder G, Biziere K, Stephane M, Dogan AS, Dannals RF, et al. Dopamine D2 and D3 receptor occupancy in normal humans treated with the antipsychotic drug aripiprazole (OPC 14597): a study using positron emission tomography and [11C]raclopride. Neuropsychopharmacology 2002;27:248–59.
Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 2000;152:174–80.
Langer O, Någren K, Dollé F, Lundkvist C, Sandell J, Swahn C-G, et al. Precursor synthesis and radiolabelling of the dopamine D2 receptor ligand [11C]raclopride from [11C]methyl triflate. J Label Compd Radiopharm 1999;42:1183–93.
Watson CC, Newport D, Casey ME, deKemp RA, Beanlands RS, Schmand M,et al. Evaluation of simulation-based scatter correction for 3D PET cardiac imaging. IEEE Trans Nucl Sci 1997;44:90–7.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imag 1994;13:601–9.
Loevinger R, Budinger TF, Watson EE. MIRD primer for absorbed dose calculations. The Society of Nuclear Medicine, New York, NY, 1991.
Cristy M, Eckerman KF. Specific absorbed fractions of energy at various ages from internal photons sources. ORNL Report ORNL/TM-8381 V1–V7. Oak Ridge, TN: Oak Ridge National Laboratory; 1987.
Stabin MG. MIRDOSE: personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 1996;37:538–46.
ICRP Publication 60: Recommendations of the International Commission on Radiological Protection. Ann ICRP 1991;21:493–502.
ICRP Publication 80: Radiation dose to patients from radiopharmaceuticals. Addendum 2 to ICRP Publication 53. Ann ICRP 1998;28:1–126.
Herscovitch P, Schmall B, Doudet DJ, Carson RE, Eckelman WC. Biodistribution and radiation dose estimates for [C-11]raclopride. J Nucl Med 1997;38:224P.
Deloar HM, Fujiwara T, Nakamura T, Itoh M, Imai D, Miyake M, et al. Estimation of internal absorbed dose of l-[methyl-11C]methionine using whole-body positron emission tomography. Eur J Nucl Med 1998;25:629–33.
Beekhuis H. Population radiation absorbed dose from nuclear medicine procedures in the Netherlands. Health Physics 1988;54:287–91.
ICRP Publication 62: Radiological protection in biomedical research. Ann ICRP 1991;22:1–28.
Billon S, Morin A, Caer S, Baysson H, Gambard JP, Rannou A, et al. Evaluation de l’exposition de la population française à la radioactivité naturelle. Radioprotection 2004;39:213–32.
Balanov MI, Krisyuk EM, Ramel C. Environmental radioactivity, population exposure and related health risks in the east Baltic region. Scand J Work Environ Health 1999;25:17–32.
Acknowledgements
This study was partially financially supported by Solvay Pharmaceuticals, Weesp, The Netherlands. We are greatly indebted to the chemical/radiopharmaceutical and nursing staff of Service Hospitalier Frédéric Joliot for the synthesis of the [11C]raclopride and patient management, respectively, and to Dr. T. Duvauchelle from ASTER, Paris, France, for his clinical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro, MJ., Ricard, M., Bourgeois, S. et al. Biodistribution and radiation dosimetry of [11C]raclopride in healthy volunteers. Eur J Nucl Med Mol Imaging 32, 952–958 (2005). https://doi.org/10.1007/s00259-005-1783-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1783-2