Abstract
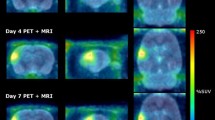
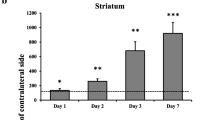
In this study we wished to determine whether technetium-99m annexin V, an in vivo marker of cellular injury and death, could be used to noninvasively monitor neuronal injury following focal middle cerebral artery (MCA) occlusion/reperfusion injury. Sixteen adult male Sprague-Dawley rats (along with four controls) underwent left (unilateral) MCA intraluminal beaded thread occlusion for 2 h followed by reperfusion. One hour following tail vein injection of 5–10 mCi of 99mTc-annexin V, animals underwent either single-photon emission computerized tomography (SPECT) or autoradiography followed by immunohistochemical analyses. There was abnormal, bilateral, multifocal uptake of 99mTc-annexin V in each cerebral hemisphere as seen by both SPECT and autoradiography at 4 h and 1, 3, and 7 days after initiation of occlusion. The average maximal annexin V uptake at 4 h was 310%±85% and 365%±151% above control values (P<0.006) within the right and left hemispheres, respectively, peaking on day 3 with values of 925%±734% and 1,194%±643% (P<0.03) that decreased by day 7 to 489%±233% and 785%±225% (P<0.01). Total lesional volume of the left hemisphere was 226%, 261%, and 451% (P<0.03) larger than the right at 4, 24, and 72 h after injury, respectively. Annexin V localized to the cytoplasm of injured neurons ipsilateral to the site of injury as well as to otherwise normal-appearing neurons of the contralateral hemisphere as confirmed by dual fluorescent microscopy. It is concluded that there is abnormal bilateral, multifocal annexin V uptake, greater on the left than on the right side, within 4 h of unilateral left MCA ischemic injury and that the uptake peaks at 3 days and decreases by 7 days after injury. This pattern suggests that neuronal stress may play a role in the response of the brain to focal injury and be responsible for annexin V uptake outside the region of ischemic insult.






Similar content being viewed by others
References
Vexler ZS, Roberts TPL, Bollen AW, Derugin N, Arieff AI. Transient cerebral ischemia. Association of apoptosis induction with hypoperfusion. J Clin Invest 1997; 99:1453–1459.
Du C, Hu R, Csernansky CA, Hsu CY, Choi DW. Very delayed infarction after mild focal cerebral ischemia: a role for apoptosis? J Cereb Blood Flow Metab 1996; 16:195–201.
Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995; 267:1456–1462.
Hara H, Friedlander RM, Gagliardini V, Ayata C, Fink K, Huang Z, Shimizu-Sasamata M, Yuan J, Moskowitz MA. Inhibition of interleukin 1β converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc Natl Acad Sci U S A 1997; 94:2007–2012.
Allen RT, Hunter WJ 3rd, Agrawal DK. Morphological and biochemical characterization and analysis of apoptosis. J Pharm Toxicol Methods 1997; 37:215–228.
Yenari MA, Palmer JT, Sun GH, de Crespigny A, Moseley ME, Steinberg GK. Time-course and treatment response with SNX-111, an N-type calcium channel blocker, in a rodent model of focal cerebral ischemia using diffusion-weighted MRI. Brain Res 1996; 739:36–45.
Wood BL, Gibson DF, Tait JF. Increased phosphatidylserine exposure in sickle cell disease: flow-cytometric measurement and clinical associations. Blood 1996; 88:1873–1880.
Larsen SK, Solomon HF, Caldwell G, Abrams MJ. [99mTc]tricine: a useful precursor complex for the radiolabeling of hydrazinonicotinate protein conjugates. Bioconjug Chem 1995; 6:635–638.
Lalush DS, Frey EC, Tsui BM. Fast maximum entropy approximation in SPECT using the RBI-MAP algorithm. IEEE Trans Med Imaging 2000;19:286–294.
D’Arceuil H, Rhine W, de Crespigny A, Yenari M, Tait JF, Strauss HW, Engelhorn T, Kastrup A, Moseley M, Blankenberg FG.99mTc annexin V imaging of neonatal hypoxic brain injury. Stroke 2000; 31:2692–2700.
Neumann-Haefelin T, Kastrup A, de Crespigny A, Yenari MA, Ringer T, Sun GH, Moseley ME. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke 2000; 31:1965–1972.
Strauss HW, Narula J, Blankenberg FG. Radioimaging to identify myocardial cell death and probably injury. Lancet 2000; 356:180–181.
Hammill AK, Uhr JW, Scheuermann RH. Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp Cell Res 1999; 251:16–21.
Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab 2000; 20:1380–1391.
Ran S, Thorpe PE. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int J Radiat Oncol Biol Phys 2002; 54:1479–1484.
Ito T, Takenaka K, Sakai H, Yoshimura S, Hayashi K, Noda S, Sakai N. Elevation of mRNA levels of tissue-type plasminogen activator and urokinase-type plasminogen activator in hippocampus and cerebral cortex following middle cerebral artery occlusion in rats. Neurol Res 2000; 22:413–419.
Kinouchi H, Huang H, Arai S, Mizoi K, Yoshimoto T. Induction of cyclooxygenase-2 messenger RNA after transient and permanent middle cerebral artery occlusion in rats: comparison with c-fos messenger RNA by using in situ hybridization. J Neurosurg 1999; 91:1005–1012.
Lennmyr F, Ata KA, Funa K, Olsson Y, Terent A. Expression of vascular endothelial growth factor (VEGF) and its receptors (Flt-1 and Flk-1) following permanent and transient occlusion of the middle cerebral artery in the rat. J Neuropathol Exp Neurol 1998; 57:874–882.
Kim Y, Truettner J, Zhao W, Busto R, Ginsberg MD. The influence of delayed postischemic hyperthermia following transient focal ischemia: alterations of gene expression. J Neurol Sci 1998; 159:1–10.
Liu JS, Chang YY, Chen WH, Chen SS. Delayed transhemispheric c-fos gene expression after focal cerebral ischemia-reperfusion in rats. J Formos Med Assoc 1995; 94:649–654.
Acknowledgements
This work was supported by The National Institutes of Health, HL-61717, NINDS, R01 NS40516 (MAY). The authors also wish to thank Bonnie Bell for her efforts in preparing histopathologic sections for routine and immunohistochemical analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Carina Mari and Murat Karabiyikoglu contributed equally to the work presented here.
Rights and permissions
About this article
Cite this article
Mari, C., Karabiyikoglu, M., Goris, M.L. et al. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging 31, 733–739 (2004). https://doi.org/10.1007/s00259-004-1473-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1473-5