Abstract
Objective
Lumbar spine MRI interpretations have high variability reducing utility for surgical planning. This study evaluated a convolutional neural network (CNN) framework that generates automated MRI grading for its ability to predict the level that was surgically decompressed.
Materials and methods
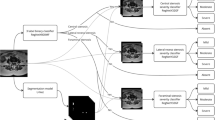
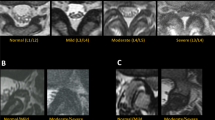
Patients who had single-level decompression were retrospectively evaluated. Sagittal T2 images were processed by a CNN (SpineNet), which provided grading for the following: central canal stenosis, disc narrowing, disc degeneration, spondylolisthesis, upper/lower endplate morphologic changes, and upper/lower marrow changes. The grades were used to calculate an aggregate score. The variables and the aggregate score were analyzed for their ability to predict the surgical level. For each surgical level subgroup, the surgical level aggregate scores were compared with the non-surgical levels.
Results
A total of 141 patients met the inclusion criteria (82 women, 59 men; mean age 64 years; age range 28–89 years). SpineNet did not identify central canal stenosis in 32 patients. Of the remaining 109, 96 (88%) patients had a decompression at the level of greatest stenosis. The higher stenotic grade was present only at the surgical level in 82/96 (85%) patients. The level with the highest aggregate score matched the surgical level in 103/141 (73%) patients and was unique to the surgical level in 91/103 (88%) patients. Overall, the highest aggregate score identified the surgical level in 91/141 (65%) patients. The aggregate MRI score mean was significantly higher for the L3-S1 surgical levels.
Conclusion
A previously developed CNN framework accurately predicts the level of microdecompression for degenerative spinal stenosis in most patients.





Similar content being viewed by others
References
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 [cited 2019 Jan 15];380:2163–2196. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0140673612617292.
Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002 [cited 2019 Jan 15];137:586–597. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12353946.
de Schepper EIT, Koes BW, Veldhuizen EFH, Oei EHG, Bierma-Zeinstra SMA, Luijsterburg PAJ. Prevalence of spinal pathology in patients presenting for lumbar MRI as referred from general practice. Fam Pract. 2016 [cited 2019 Jan 15];33:51–56. Available from: https://academic.oup.com/fampra/article-lookup/doi/10.1093/fampra/cmv097.
Chou R, Deyo RA, Jarvik JG. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin North Am. 2012 [cited 2019 Jan 15] ;50:569–585. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22643385.
Herzog R, Elgort DR, Flanders AE, Moley PJ. Variability in diagnostic error rates of 10 MRI centers performing lumbar spine MRI examinations on the same patient within a 3-week period. Spine J. 2017 [cited 2019 Jan 15];17:554–561. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1529943016310932.
Imaad-ur-Rehman, Hamid RS, Akhtar W, Shamim MS, Naqi R, Siddiq HI. Observer variation in MRI evaluation of patients with suspected lumbar disc herniation and nerve root compression: comparison of neuroradiologist and neurosurgeon’s interpretations. J Pak Med Assoc. 2012 [cited 2019 Jan 16];62:826–829. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23862259.
Al-Tameemi HN, Al-Essawi S, Shukri M, Naji FK. Using magnetic resonance myelography to improve interobserver agreement in the evaluation of lumbar spinal canal stenosis and root compression. Asian Spine J. 2017 [cited 2019 Jan 16];11:198. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28443163.
Marawar S V., Madom IA, Palumbo M, Tallarico RA, Ordway NR, Metkar U, et al. Surgeon reliability for the assessment of lumbar spinal stenosis on MRI: the impact of surgeon experience. Int J Spine Surg. 2017 [cited 2019 Jan 16];11:34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29372138.
van Rijn JC, Klemetsö N, Reitsma JB, Majoie CBLM, Hulsmans FJ, Peul WC, et al. Observer variation in MRI evaluation of patients suspected of lumbar disk herniation. AJR Am J Roentgenol. 2005 [cited 2019 Jan 15];184:299–303. Available from: http://www.ajronline.org/doi/10.2214/ajr.184.1.01840299.
Steurer J, Roner S, Gnannt R, Hodler J, LumbSten Research Collaboration. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011 [cited 2019 Jan 16];12:175. Available from: http://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/1471-2474-12-175.
Larson DB, Chen MC, Lungren MP, Halabi SS, Stence N V., Langlotz CP. Performance of a deep-learning neural network model in assessing skeletal maturity on pediatric hand radiographs. Radiology. 2018 [cited 2019 may 31];287:313–322. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29095675.
Ye H, Gao F, Yin Y, Guo D, Zhao P, Lu Y, et al. Precise diagnosis of intracranial hemorrhage and subtypes using a three-dimensional joint convolutional and recurrent neural network. Eur Radiol. 2019 [cited 2019 May 31]; Available from: http://link.springer.com/10.1007/s00330-019-06163-2.
Trebeschi S, van Griethuysen JJM, Lambregts DMJ, Lahaye MJ, Parmar C, Bakers FCH, et al. Deep learning for fully-automated localization and segmentation of rectal cancer on multiparametric MR. Sci Rep. 2017 [cited 2019 May 31];7:5301. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28706185.
Walsh SLF, Calandriello L, Silva M, Sverzellati N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: a case-cohort study. Lancet Respir Med. 2018 [cited00202019 May 31];6:837–845. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213260018302868
Liu F, Zhou Z, Samsonov A, Blankenbaker D, Larison W, Kanarek A, et al. Deep learning approach for evaluating knee MR images: achieving high diagnostic performance for cartilage lesion detection. Radiology [Internet]. 2018 [cited 2019 May 31];289:160–169. Available from: http://pubs.rsna.org/doi/10.1148/radiol.2018172986
Sharma K, Rupprecht C, Caroli A, Aparicio MC, Remuzzi A, Baust M, et al. Automatic segmentation of kidneys using deep learning for total kidney volume quantification in autosomal dominant polycystic kidney disease. Sci Rep [Internet]. 2017 [cited 2019 may 31];7:2049. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28515418.
Lucas M, Jansen I, Savci-Heijink CD, Meijer SL, de Boer OJ, van Leeuwen TG, et al. Deep learning for automatic Gleason pattern classification for grade group determination of prostate biopsies. Virchows Arch [Internet]. 2019 [cited 2019 May 31]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/31098801.
Jamaludin A, Lootus M, Kadir T, Zisserman A, Urban J, Battié MC, et al. ISSLS PRIZE IN BIOENGINEERING SCIENCE 2017: automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. Eur Spine J [Internet]. 2017 [cited 2019 Jan 16];26:1374–83. Available from: http://link.springer.com/10.1007/s00586-017-4956-3
Jamaludin A, Kadir A, Zisserman A, Urban A, Fairbank J, Williams F. Adapting a deep learning model to a different grading system in a new dataset. Baniff: Int Soc Study Lumbar Spine Annu Meet; 2018.
Kadir T, Zisserman A, Fairbank J, Jamaludin A, Urban J. SpineNet: automated vertebra and disc gradings using deep learning. Radiol Soc North Am Annu Meet. Chicago, IL; 2018.
Lu J-T, Pedemonte S, Bizzo B, Doyle S, Andriole KP, Michalski MH, et al. DeepSPINE: automated lumbar vertebral segmentation, disc-level designation, and spinal stenosis grading using deep learning. Proc Mach Learn Res. 2018 [cited 2019 May 31];85:403–419. Available from: http://arxiv.org/abs/1807.10215
Ishimoto Y, Jamaludin A, Cooper C, Walker-Bone K, Yamada H, Hashizume H, et al. Could automated machine-learned MRI grading aid epidemiological studies of lumbar spinal stenosis? Validation within the Wakayama spine study. BMC Musculoskelet Disord [Internet]. BioMed Central Ltd.; 2020 [cited 2020 May 8];21:158. Available from: https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-020-3164-1
Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer [Internet]. NIH Public Access; 2018 [cited 2019 may 31];18:500–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29777175.
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) [Internet]. 2001 [cited 2019 Jan 16];26:1873–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11568697.
Finkenstaedt T, Del Grande F, Bolog N, Ulrich NH, Tok S, Burgstaller JM, et al. Correlation of listhesis on upright radiographs and central lumbar spinal canal stenosis on supine MRI: is it possible to predict lumbar spinal canal stenosis? Skeletal Radiol [Internet]. 2018 [cited 2019 may 30];47:1269–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29651713.
Jönsson B, Annertz M, Sjöberg C, Strömqvist B. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part I: clinical features related to radiographic findings. Spine (Phila Pa 1976) [Internet]. 1997 [cited 2019 may 30];22:2932–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9431629.
Bechara BP, Agarwal V, Boardman J, Perera S, Weiner DK, Vo N, et al. Correlation of pain with objective quantification of magnetic resonance images in older adults with chronic low back pain. Spine (Phila Pa 1976) [Internet]. 2014 [cited 2019 may 29];39:469–75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24384652.
Ziegler DS, Carreon L, Andersen MO, Jensen RK. The Association between preoperative MRI findings and surgical revision within three years after surgery for lumbar disc herniation. Spine (Phila Pa 1976) [Internet]. 2019 [cited 2019 may 29];44:818–25. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30475335.
Takatalo J, Karppinen J, Näyhä S, Taimela S, Niinimäki J, Blanco Sequeiros R, et al. Association between adolescent sport activities and lumbar disk degeneration among young adults. Scand J Med Sci Sports [Internet]. 2017 [cited 2019 may 29];27:1993–2001. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28075521.
Takatalo J, Karppinen J, Niinimäki J, Taimela S, Näyhä S, Mutanen P, et al. Does lumbar disc degeneration on magnetic resonance imaging associate with low back symptom severity in young Finnish adults? Spine (Phila Pa 1976) [Internet]. 2011 [cited 2019 may 29];36:2180–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21358475.
Hancock MJ, Kjaer P, Kent P, Jensen RK, Jensen TS. Is the number of different MRI findings more strongly associated with low back pain than single MRI findings? Spine (Phila Pa 1976) [Internet]. 2017 [cited 2019 Aug 21];42:1283–8. Available from: http://insights.ovid.com/crossref?an=00007632-201709010-00008
Covaro A, Vilà-Canet G, de Frutos AG, Ubierna MT, Ciccolo F, Caceres E. Management of degenerative lumbar spinal stenosis: an evidence-based review. EFORT open Rev [Internet]. British Editorial Society of Bone and Joint Surgery; 2016 [cited 2019 Jan 21];1:267–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28461958.
Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg [Internet]. Frontiers Media SA; 2016 [cited 2019 Jan 21];3:19. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27092304.
Kjaer P, Leboeuf-Yde C, Korsholm L, Sorensen JS, Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine (Phila Pa 1976) [Internet]. 2005 [cited 2019 Jan 21];30:1173–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15897832.
Rahme R, Moussa R. The modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am J Neuroradiol. 2008 [cited 2019 Jan 21];29:838–842. Available from: http://www.ajnr.org/lookup/doi/10.3174/ajnr.A0925
Xiao L, Ni C, Shi J, Wang Z, Wang S, Zhang J, et al. Analysis of correlation between vertebral endplate change and lumbar disc degeneration. Med Sci Monit. 2017 [cited 2019 Feb 6];23:4932–4938. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29032381.
Guo R, Yang X, Zhong Y, Lai Q, Gao T, Lai F, et al. Correlations between Modic change and degeneration in 3-joint complex of the lower lumbar spine: A retrospective study. Medicine (Baltimore) [Internet]. Wolters Kluwer Health; 2018 [cited 2019 Jan 21];97:e12496. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30235755.
Hayashi T, Daubs MD, Suzuki A, Scott TP, Phan KH, Ruangchainikom M, et al. Motion characteristics and related factors of Modic changes in the lumbar spine. J Neurosurg Spine. 2015 [cited 2019 Jan 21];22:511–517. Available from: https://thejns.org/view/journals/j-neurosurg-spine/22/5/article-p511.xml
Laudato PA, Bartanusz V. What is the role of surgery in low back pain associated with Modic changes?. Rev Med Suisse. 2017 [cited 2019 Jan 21];13:1292–1295. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28643966.
Keller A, Boyle E, Skog TA, Cassidy JD, Bautz-Holter E 2012. Are Modic changes prognostic for recovery in a cohort of patients with non-specific low back pain? Eur Spine J . Springer; [cited 2019 Jan 21];21:418–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21837412.
Tonosu J, Oka H, Higashikawa A, Okazaki H, Tanaka S, Matsudaira K. The associations between magnetic resonance imaging findings and low back pain: a 10-year longitudinal analysis. Espinoza Orías AA, editor. PLoS One [Internet]. 2017 [cited 2019 Feb 6];12:e0188057. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29141001.
Bhalla A, Cha TD, Weber C, Nerland U, Gulati S, Lønne G. Decompressive surgery for lumbar spinal stenosis across the Atlantic: a comparison of preoperative MRI between matched cohorts from the US and Norway. Acta Neurochir (Wien) [Internet]. 2018 [cited 2019 Jan 16];160:419–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29350291.
Nerland US, Jakola AS, Solheim O, Weber C, Rao V, Lønne G, et al. Minimally invasive decompression versus open laminectomy for central stenosis of the lumbar spine: pragmatic comparative effectiveness study. BMJ [Internet]. BMJ Publishing Group; 2015 [cited 2019 Jan 21];350:h1603. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25833966.
Nerland US, Jakola AS, Solheim O, Weber C, Rao V, Lønne G, et al. Comparative effectiveness of microdecompression and laminectomy for central lumbar spinal stenosis: study protocol for an observational study. BMJ Open [Internet]. 2014 [cited 2019 Feb 6];4:e004651. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24650809.
Yuan S, Zou Y, Li Y, Chen M, Yue Y. A clinically relevant MRI grading system for lumbar central canal stenosis. Clin Imaging [Internet]. 2016 [cited 2019 Feb 6];40:1140–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27519125.
Cheung KMC, Karppinen J, Chan D, Ho DWH, Song Y-Q, Sham P, et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) [Internet]. 2009 [cited 2019 Feb 6];34:934–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19532001.
Acknowledgments
The authors would like to acknowledge Timor Kadir, PhD (University of Oxford, UK), for his efforts to develop SpineNet and guide the use of the MRI grading tool for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
An Institutional Review Board protocol (IRB #42783, Wake Forest University Health Sciences) was obtained, allowing for retrospective review of patients with lumbar spinal stenosis treated with surgery.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roller, B.L., Boutin, R.D., O’Gara, T.J. et al. Accurate prediction of lumbar microdecompression level with an automated MRI grading system. Skeletal Radiol 50, 69–78 (2021). https://doi.org/10.1007/s00256-020-03505-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-020-03505-w