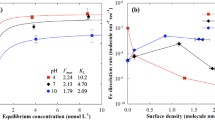
Abstract
Bolar earths deposits from Mt Amiata (Central Italy) consist of nanosized pseudo-spherical goethite, with average crystal size of 10–15 nm (as determined by X-ray powder diffraction and transmission electron microscopy observations), possibly associated to amorphous silica and minor sheet silicates, quartz and feldspars. Chemical analyses revealed high As contents (up to 7.4 wt% As2O5), thus indicating the occurrence of a potentially dangerous contaminant. Arsenic doesn’t occur as a specific As phase, but it is strictly associated with goethite nanocrystals. Eh and pH measurements suggest that As occurs as arsenate anions (H2AsO −4 and HAsO 2−4 ), which are easily and strongly adsorbed to goethite surfaces. The high specific surface area, resulting from goethite nanosize, and the absence of competitive anions explain the extremely efficient adsorption of arsenate and the anomalously high As content in bolar earths. Overall physical/chemical data suggest stable arsenate adsorption, with very limited risk for As release to the environment.







Similar content being viewed by others
References
Antelo J, Avena M, Fiol S, López R, Arce F (2005) Effects of pH and ionic strength on the adsorption of phosphate and arsenate at the goethite–water interface. J Colloid Interface Sci 285:476–486
Brogi A (2004) Miocene low-angle detachments and upper crust megaboudinage in the Mt. Amiata geothermal area (Northern Apennines, Italy). Geodin Acta 17(6):375–387
Carlson L, Bigham JM, Schwertmann U, Kyek A, Wagner F (2002) Scavenging of As from acid mine drainage by schwertmannite and ferrihydrite: a comparison with synthetic analogues. Environ Sci Technol 36:1712–1719
Cliff G, Lorimer GW (1975) The quantitative analysis of thin specimens. J Microsc 103:203–207
Cornell RM, Schwertmann U (2003) The iron oxides. Structure, properties, reactions, occurrence and uses, 2nd edn. Wiley, Weinheim, Germany, p 663
Cuteri F, Mascaro I (1995) Colline metallifere. Inventario del patrimonio minerario e mineralogico. In: Regione Toscana (ed) Aspetti naturalistici e storico-archeologici, vol 1. Firenze p 182
Dixit S, Hering J (2003) Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37:4182–4189
Dzombak DA, Morel FM (1990) Surface complexation modelling: hydrous ferric oxide. Wiley, New York
Fendorf S, Eick M, Grossl P, Sparks D (1997) Arsenate and chromate retention mechanism on goethite. 1. Surface structure. Environ Sci Technol 31:315–320
Ferrari L, Ponticelli S, Burlamacchi L, Manetti P (1996) Volcanological evolution of the Monte Amiata, southern Tuscany: new geological and petrochemical data. Acta Vulcanol 8:41–56
Gao Y, Mucci A (2001) Acid base reactions, phosphate and arsenate complexation, and their competitive adsorption at the surface of goethite in 0.7 M NaCl solution. Geochim Cosmochim Acta 65:2361–2378
Garcia-Sanchez A, Alvarez-Ayuso E, Rodriguez-Martin F (2002) Sorption of As (V) by some oxyhydroxides and clay minerals. Application to its immobilization in two polluted mining soils. Clay Miner 37:187–194
Gräfe M, Eick MJ, Grossl PR (2001) Adsorption of arsenate (V) and arsenite (III) on goethite in the presence and absence of dissolved organic carbon. Soil Sci Soc Am J 65(6):1680–1687
Grossl PR, Eick M, Sparks DL, Goldberg S, Ainsworth CC (1997) Arsenate and chromate retention mechanisms on goethite. 2. Kinetic evaluation using a pressure-jump relaxation technique. Environ Sci Technol 31:321–326
Hiemstra T, Van Riemsdijk WH (1999) Surface structural ion adsorption modelling of competitive binding of oxyanions by metal (hydr)oxides. J Colloid Interface Sci 210:182–193
Loppi S (2000) Lichen biomonitoring as a tool for assessing air quality in geothermal areas. In: Iglesis E, Blackwell D, Hunt T, Kund J, Tamanyu S, Kimbara K (eds) Proceedings of the World geothermal congress 2000, Kyushu-Tohoku, Japan
Lotti B (1910) Geologia della Toscana. Mem Descr Carta Geol Ital XIII:482
Lumsdon DO, Evans LJ (1994) Surface complexation model parameters for goethite (FeOOH). J Colloid Interface Sci 164:119–125
Manasse E (1915) Sulla composizione chimica delle terre gialle e bolari del Monte Amiata. Atti Soc Tosc Sci Nat Mem 30:101–119
Manasse A, Mellini M (2006) Iron (hydr)oxide nanocrystals in raw and burnt sienna pigments. Eur J Mineral (in press)
Manning BA, Fendorf SE, Goldberg S (1998) Surface structures and stability of arsenic (III) on goethite: spectroscopic evidence for inner-sphere complexes. Environ Sci Technol 32:2383–2388
Mantelli F, Salutini A, Grilli Ciclioni A, Bucci P, Carrozzino S, Bozzelli M (1999) Presenza di arsenico nelle acque di acquedotto e nelle fonti di approvvigionamento idrico in Toscana. Quaderni di Geologia Applicata 2:271–281
Mascaro I, Benvenuti M, Corsini F, Costagliola P, Lattanzi P, Parrini P, Tanelli G (2001) Mine wastes at the polymetallic deposit of Fenice Capanne (southern Tuscany, Italy). Mineralogy, geochemistry and environmental impact. Environ Geol 41:417–429
Mellini M, Menichini R (1985) Proportionality factors for thin film TEM/EDS microanalysis of silicate minerals. Rend Soc Ital Mineral Petrol 40:261–266
Minissale A, Magro G, Vaselli O, Verrucchi C, Perticone I (1997) Geochemistry of water and gas discharges from the Mt. Amiata silicic complex and surrounding areas (Central Italy). J Volcanol Geotherm Res 79:223–251
O’Reilly SE, Strawn DG, Sparks DL (2001) Residence time effects on arsenate adsorption/desorption mechanisms on goethite. Soil Sci Soc Am J 65:67–77
Protano G, Riccobono F, Sabatini G (1998) Geochemical maps of Southern Tuscany. Construction criteria and environmental relevance through the Hg, As, S, Pb, Cd examples. Mem Descr Carta Geol Ital LV:109–140
Sherman DM, Randall SR (2003) Surface complexation of arsenic (V) to iron (III) (hydr)oxides: structural mechanism from ab initio molecular geometries and EXAFS spectroscopy. Geochim Cosmochim Acta 67:4223–4230
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Sun X, Doner HE (1996) An investigation of arsenate and arsenite bonding structures on goethite by FTIR. Soil Sci 161:865–872
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci Total Environ 327:41–51
Waychunas GA, Davis JA, Fuller CC (1995) Geometry of sorbed arsenate on ferrihydrite and crystalline FeOOH: reevaluation of EXAFS results and topological factors in prediction sorbate geometry and evidence for monodentate complexes. Geochim Cosmochim Acta 59:3655–3661
Webster JG, Nordstrom DK (2003) Geothermal arsenic—the source, transport and fate of arsenic in geothermal systems. In: Welch AH, Stollwerk KG (eds) Arsenic in groundwater. Kluwer Academic Publishers, Boston, pp 101–125
Acknowledgement
The present research has been funded by the PRIN 2004 project on “Mineralogia delle fasi responsabili della mobilizzazione e rimozione dell’arsenico: implicazioni ambientali.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manasse, A., Viti, C. Arsenic adsorption on nanocrystalline goethite: the natural example of bolar earths from Mt Amiata (Central Italy). Environ Geol 52, 1365–1374 (2007). https://doi.org/10.1007/s00254-006-0579-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0579-4