Abstract
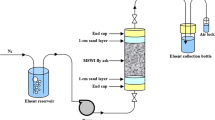
The maximum concentration of the majority of the trace metals in the leachates from shake and column test of lignite fly ash (LFA) was within the prescribed limits; however, total dissolved solids, total hardness, cations and anions (except K+), being above the prescribed limits, may lead to the increase in the hardness and salinity in the soil on the disposal of LFA. Present generation of huge amount of fly ash from thermal power plants (TPPs) is a big challenge concerning contamination of soil, crop produce and surface and ground water bodies due to the presence of some of the toxic trace metals in it. The leaching behavior of alkaline LFA (pH, 10.94), from TPP of Neyveli Lignite Corporation (NLC), India, was investigated by shake and column tests using water and sodium acetate buffer. The leaching of trace metals from LFA was governed by their concentrations, association with the ash particles, leaching duration and pH of the leachate (most influencing parameter). The leaching of metals followed the order: buffer column > aqueous column > aqueous shake > buffer shake test.






Similar content being viewed by others
References
Bauer CF, Natush DFS (1981) Identification and quantitation of carbonate compounds in coal fly ash. Environ Sci Technol 15:783–7880
Bernhard M, Brinkman FE, Sadler PJ (1986) The importance of chemical speciation in environmental processes, Dohlem Kon Feremen. Springer, Berlin Heidelberg New York
Biermann AH, Ondow JM (1980) Application of surface depositions models to size-fractinated coal fly ash. Atmos Environ 14:289
Bradley J, Shyam A, Reeuwijk LB, Zevenberges (1999) Clay formation during weathering of alkaline coal fly ash. In: Proceedings of the international ash utilization symposium and the world of coal ash conference, Lexington
Brown TH, Bland Wheeldn JM (1997) Pressurized fluidized bed combusion ash: 2. soil and mine spoil amendment use options. Fuel 76:741–748
Chaddha G, Seera MSJ (1983) Magnetic components and particle size distribution of coal fly ash. J Phys D Appl Phys 16:1767–1776
Chong SL, Peart J, Ormsby WC (1990) Griffith MS Leaching test studies using extraction procedure toxicity test and toxicity characteristic leaching procedure. Public Roads 54:241
Claudia B, Stefania B, Morabite R (1999) Int J Environ Anal Chem 75:19
Council of the European Communities (1991) Proposal for a Council Directive on the landfill. Off J Eur Commun 34(C 190):1
Davidson RL, Natusch DFS, Wallace JR, Evans CA (1974) Trace elements in fly ash-dependence of concentration on particle size. Environ Sci Technol 8:1107–1113
Drakonaki S, Diamadopoulos E, Vamvouka D, Lahaniatis M (1998) Leaching behavior of lignite fly ash. J Environ Sci Health A 33(2):237–248
Drinking Water Threshold Values in U.K. (2001) (Derived from Guidance Note, CI RCI Note 59/83). In: Sears LKA (ed) Properties and use of coal fly ash: a valuable industrial by-product. Thomas Telford Publishing Thomas Telford Ltd., London, pp 30–62
Dunn-Rankin D, Kerstein A (1987) Numerical simulation of particle size distribution evolution during pulverized coal combustion. Combust Flame 69:193–209
EA-3236 (1993) Physico-chemical characteristics of utility solid waste. Electric Power Research Institute, Palo Alto
EPA (1984) Ground water protection strategy. USEPA Office of Ground-Water Protection, Washington
Ecke H, Menad N, Lagerkvist Mater AJ (2002) Cycles Waste Manage 4:117
Egemen E, Yurteri C (1996) Regulatory leaching tests for fly ash: a case study regulatory leaching tests for fly ash: a case study. Waste Manage Res 14:43–50
Elseewi AA, Page AL, Grimm SR (1980) Chemical characterization of fly ash aqueous system. J Environ Qual 9:224
Fernandez-Turiel JL, de Carvalho W, Cabanas M, Querol X, Lopez-Soler A (1994) Mobility of heavy metals from coal fly ash. Environ Geol 23: 264–270
Fulekar MH (1993) The pH effects on leachability of fly-ash heavy metals: laboratory experiment. Indian J Environ Prot 13:185–192
Fulekar MH, Dave JM (1989) Leaching of fly ash constituients along stream bed flow to Yamuna river, New Delhi, India. Indian J Environ Prot 9:773
Furuya K, Miyajima Y, Chiba T, Kikuchi T (1987) Environ Technol 21:898
Grand K, Weymouth JH (1962) The nature of inorganic deposits formed during the use of Victorian Brown Coal in large industrial boilers. J Inst Fuel 35:154–160
Hansen LD, Silberman D, Fisher D, Fisher GL, Etaough DJ (1984) Chemical speciation of elements in stack-collected, respirable-size, coal fly ash. Environ Sci Technol 18:181–186
Hassett DJ, Debra F, Pflughoeft-Hassett L, Heebink V (2005) Leaching of CCBs: observations from over 25 years of research. Fuel 84:1378–1383
Henery WM, Knapp KT (1980) Compound forms of fossil fuel fly ash emissions. Environ Sci Technol 4:450–456
IS (1964) Indian Standard for Water Analysis. IS: 3025, 1964
IS (1969) Indian Standards for Ash Analysis. IS: 1350 (Part I), 1969
IS (1974) Indian Standards for Industrial Effluents. IS: 2490, 1974
IS (1983) Indian Standards for Drinking Water. IS: 10500, 1983
Iwashita A, Sakaguehi Y, Nakajima T, Takanashi H, Ohki A, Kambara S (2005) Leaching characteristics of boron and selenium for various coal fly ashes. Fuel 84:479–485
Iyer RS (2002) The surface chemistry of leaching coal fly ash, J Hazard Mater B93:321–329
Jankowski J, Ward CR, Frech D, Groves S (2006) Mobility of trace elements from selected Australian fly ashes and its potential impacts on aquatic ecosystems. Fuel 85:243–256
Karuppiah M, Gupta G (1997) Toxicity of metals in coal combustion ash leachates. J Hazard Mater 56:53–58
Khanra S, Mallick D (1998) Studies on the phase mineralogy and leaching characteristics of coal fly ash. Water Air Soil Pollut 10:251–275
Killingley J, McEvoy S, Dokumeu C, Stanber J, Dale L (2000) Trace element leaching from fly ash from Australian power stations End of grant report, Australian coal association research program, project C8051, CSIRO division of energy technology, pp 98
Kim JB, Lee WK (1997) Leaching characteristic of heavy metals from ashes discharged from MSWI. J KSEE 19:481–490
Kukier U, Ishak CF, Summer ME, Muller WP (2003) Composition and element solubility of magnetic and non-magnetic fly ash fractions. Environ Pollut 123:255–266
Lee S, Kahn JJ (1997) Environ Health A33:649
Lindon KAS (2001) The properties and use of coal fly ash: a valuable industrial product. In: Lindon KAS (ed) Fly ash and the environment, ISBN: 07277 30150. Thomas Telford Publishing, Thomas Telford Ltd., London
Mattigod SV, Eary LE, Dhanpal R (1990) Geochemical factors controlling the mobilisation of inorganic constituents from fossil fuel combustion residues 1: review of major elements. J Environ Qual 191:188–201
Meserole FB, Schnitzgebel K, Magee Mann RM (1979) Trace elements emission from coal filed power plants. J Environ Pollut 101:620–624
Mitchell RS, Gluscoter HJ (1976) Mineralogy of ash of some American coals: variations with temperature and source. Fuel 55:90–96
Moreno N, Querol X, Andres JM, Stanton Towler M, Nugteren H, Janssen-Jurkovicova M, Jone R (2005) Physico-chemical characteristics of European pulverized coal combustion fly ashes. Fuel 84:1351–1363
Mukherjee AB, Zevenhoven R (2006) Mercury in coal ash and its fate in the Indian sub-continent: a synoptic review. Sci Total Environ (In press)
Murarka L, Rai PD, Ainsworth CC (1992) ASTM specification technical publishing STP 1075 (Waste test quality assurance vol 3.) Chem Abstr 17:219027
Natusch DFS, Wallace JR, Ewans Jr CA (1974) Toxic trace elements: preferential concentration is repairable particles. Science 183:202–204
Pande SP, Hasan MJZ (1983) Inst Eng India 63:53
Pires M, Tiexeira EC (1992) Geochemical distribution of trace elements in Leao coal, Brazil. Fuel 71:1093–1096
Praharaj T, Powell MA, Hart BR, Tripaty S (2002) Leachability of elements from sub-bituminous coal fly ash from India. Environ Int 27:609–615
Querol X, Fernandez-Turiel JL, Lopez-Soler A (1995) Trace elements in coal and their behavior during combustion in a large power station. Fuel 74:331–343
Querol X, Umana JC, Alastuey A, Bertrana C, Lopez-Soler A, Plana F (2000) Energy Source 22:733–750
Querol X, Umana JC, Alastuey A, Ayora C, Lopez S, Plana F (2001) Extraction of soluble major and trace elements from fly ash in open and closed leaching systems. Fuel 80:801–813
Rajasekhar C (1996) Retention and permeability characteristics of clays and clays-fly ash systems subjected to flow of contaminants. Ph.D. thesis, Indian Institute of Science
Ram LC, Tripathi PSM, Mishra SP (1995) Moessbauer spectroscopic characterization and studies on their transformations of iron-bearing minerals during combustion of coals: correlation with fouling and slagging. Fuel Process Technol 42:47–60
Ram LC, Tripathi PSM, Mishra SP (1996) In: Proceedings of the international symposium on coal science technology industry business environment, Dhanbad, pp 38
Ram LC, Srivastava NK, Das MC, Singh G (1999) Leaching behavior of fly ash under simulated conditions vis-à-vis quality of the leachate. In: Ram LC et al (eds) Proceedings of the national seminar on bulk utilization of fly ash in agriculture and for value-added products. Dhanbad (India), ISBN 81–7525-184-0
Ram LC, Srivastava NK, Singh G (2000) Prediction of leaching behavior of TPP ash under simulated condition by column studies. In: Proceedings of the international conference on fly ash disposal and utilization (CBIP), New Delhi technical session-IV-3, pp 16
Ramesh A, Kozinski JA (2001) Investigations of ash topography/morphology and their relationship with heavy metals leachability. Environ Pollut 111:255–262
Robert CB, Jeff D (1995) Systematic errors in the use of loss-on-ignition to measure unburned carbon in fly ash. Fuel 74:570–574
Saikia N, Kato S, Kojima T (2006) Composition and leaching behavior of combustion residues. Fuel 85:264–271
Sanchez J, Tiexeira EC, Fernandes I, Pestana MHD, Machado R (1994) Geochim Brasil 8:1
Sandelin K, Backman R (2001) Trace elements in two pulverized coal-fired power stations. Environ Sci Technol 35:826–834
Seferinoglu M, Paul M, Sendstorm A, Koker A, Toprak S, Paul J (2003) Acid leaching of coal and coal-ashes. Fuel 82:1721–1724
Shim YS, Rhee SW, Lee WK (2005) Comparison of heavy metals from bottom and fly ashes in Korea and Japan. Waste Manage 25:473–480
Shuey RT (1975) Semi-conducting ore minerals. Elsevier Science, New York
Sloss L (1996) Residues from advanced coal-use technologies. IEA Coal Research, London
Smith RD, Campbell JA, Nelson KK (1979) Concentration dependence upon particle size of volatilizer elements in fly ash. Environ Sci Technol 13:553–558
Smith IM (1987) Trace elements from coal combustion:emissions, chap 2, Source of trace elements, IEACR/01 IEA Coal Research, London
Smith IM (1990) Management of AFBS Residues. IEACR/21, IEA Coal Research, London
Spears DA, Tarazona MRM, Lee SS (1994) Pyrite in U.K. Coal: its environmental significance. Fuel 73:1051–1055
Stumm W (1992) Chemistry of the solid-water interface. Wiley, New York
Swaine DJ (1977) Trace elements in coal. In: Hemphill DD (ed) Trace substances in environmental Health-XI. University of Missouri, Columbia pp 107–116
Swaine DJ (1994) Trace elements in coal and their dispersal during combustion. Fuel Process Technol 39:121–137
Theis TL, Wirth JL (1977) Sorptive behavior of trace metals on fly ash in aqueous systems. Environ Sci Technol 11:1096–1100
Tiexeira M, Samama ECJ, Brun A (1992) Environ Technol 13:995
Trivedi PR, Raj G (1992) Environmental water and soil analysis, Akashdeep Publishing House, New Delhi
Turner RR (1981) Oxidative state of arsenic in coal ash leachate. Environ Sci Technol 15:1062–1066
USEPA (United States Environmental Protection Agency) (1987) Characterization of municipal waste combustor ashes and leachates from municipal solid waste landfills and co-disposal sites, I-VI, 530-SW-87–028A-E. Washington
Van der Sloot HA (1995) Development in evaluating environmental impact from utilisation of bulk inert using lab leaching tests and verification. In: International symposium on bulk industrial waste an opportunity for use, Leeds (UK), September 1995, Report No. ECN-RX-(5-056), P34, Nov 1995
Van der Sloot HA, Piepers O, Kok AA (1984) Standard leaching test for combustion residues. In: Petten ECN (ed) Bureau for energy research projects, Netherlands Energy Research Foundation. The Netherlands, p 34
Van der Sloot HA, Heelmar TH, Aalberts G, Wahlstrom FAM (1993) In: Petten ECN (ed) Proposed leaching test for granular solid wastes. ECN-C-93–012, Ground water. Netherlands Energy Research Foundation, The Netherlands
Vassilev SV, Vassileva CG, Karayigit AI, Bulut Y, Alastuey A, Querol X (2005) Phase mineral and chemical composition of fractions separated from composite fly ashes at the Soma Power Station, Turkey. Int J Coal Geol 61:65–85
Weintraub M, Goldbery S, Orning AA (1961) A study of sulfur reactions in furnace deposits. Trans ASME J Eng Power Ser A 83:444–450
Zacharia JS (1990) Use of batch and column methodologies to assess utility wastes leaching and surface chemical attenuation. In: Report to EPRI, Palo Alto, CA, EN 73/3, pp 3–5, B1–B14
Zevenbergen C, Vander WT, Bradley JP, van der Broeck P, Orbons AJ, Van Recuwijk LP (1994) Hazard Waste Hazard Mater 11:371
Acknowledgments
The authors thank the authorities of NLC, India, for providing financial assistance. They are also thankful to Dr. S. C. Roy, Director, CFRI, for constant encouragement, providing necessary facilities for carrying out this work and for permission to publish this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ram, L.C., Srivastava, N.K., Tripathi, R.C. et al. Leaching behavior of lignite fly ash with shake and column tests. Environ Geol 51, 1119–1132 (2007). https://doi.org/10.1007/s00254-006-0403-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00254-006-0403-1