Abstract.
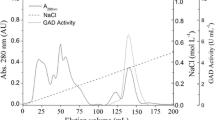
Glutamate dehydrogenase (GDH) was purified and characterized from an aerobic hyperthermophilic archaeon Aeropyrum pernix (A. pernix) K1. The enzyme has a hexameric structure with a native molecular mass of about 285±15 kDa. It was specific for NADP and thermostable (74% activity was remained after 5 h incubation at 100 °C). The activity of the enzyme increased in the presence of polar water-miscible organic solvents such as acetonitrile, methanol, and ethanol. The N-terminal sequence of GDH is Met-Gln-Pro-Thr-Asp-Pro-Leu-Glu-Glu-Ala. This sequence, except for the methionine, corresponds to amino acids 7–15 of the open reading frame (ORF) encoding the predicted GDH (ORF APE 1386). In the ORF nucleotide sequence, the codon TTG appears at the position of the methionine, suggesting that the leucine codon might be recognized as an initiation codon and translated to methionine in A. pernix GDH.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received revision: 17 November 2000
Electronic Publication
Rights and permissions
About this article
Cite this article
Helianti, .I., Morita, .Y., Yamamura, .A. et al. Characterization of native glutamate dehydrogenase from an aerobic hyperthermophilic archaeon Aeropyrum pernix K1. Appl Microbiol Biotechnol 56, 388–394 (2001). https://doi.org/10.1007/s002530100575
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002530100575