Abstract
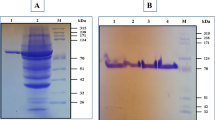
The extremely thermophilic archaeon Thermococcus hydrothermalis, isolated from a deep-sea hydrothermal vent in the East Pacific Rise at 21°N, produced an extracellular pullulanase. This enzyme was purified 97-fold to homogeneity from cell-free culture supernatant. The purified pullulanase was composed of a single polypeptide chain having an estimated molecular mass of 110 kDa (gel filtration) or 128 kDa (sodium dodecyl sulfate/polyacryl amide gel electrophoresis). The enzyme showed optimum activity at pH 5.5 and 95 °C. The thermostability and the thermoactivity were considerably increased in the presence of Ca2+. The enzyme was activated by 2-mercaptoethanol and dithiothreitol, whereas N-bromosuccinimide and α-cyclodextrin were inhibitors. This enzyme was able to hydrolyze, in addition to the α-1,6-glucosidic linkages in pullulan, α-1,4-glucosidic linkages in amylose and soluble starch, and can therefore be classified as a type II pullulanase or an amylopullulanase. The purified enzyme displayed Michaelis constant (K m) values of 0.95 mg/ml for pullulan and 3.55 mg/ml for soluble starch without calcium and, in the presence of Ca2+, 0.25 mg/ml for pullulan and 1.45 mg/ml for soluble starch.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 19 November 1997 / Received revision: 9 March 1998 / Accepted: 14 March 1998
Rights and permissions
About this article
Cite this article
Gantelet, H., Duchiron, F. Purification and properties of a thermoactive and thermostable pullulanase from Thermococcushydrothermalis, a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Appl Microbiol Biotechnol 49, 770–777 (1998). https://doi.org/10.1007/s002530051245
Issue Date:
DOI: https://doi.org/10.1007/s002530051245