Abstract
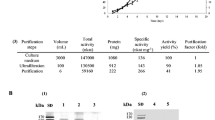
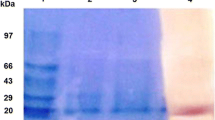
Pyranose 2-oxidase (P2O) was purified 43-fold to apparent homogeneity from the basidiomycete Phanerochaete chrysosporium using liquid chromatography on phenyl Sepharose, Mono Q (twice) and phenyl Superose. The native enzyme has a molecular mass of about 250 kDa (based on native PAGE) and is composed of four identical subunits of 65 kDa. It contains three isoforms of isoelectric point (pI) 5.0, 5.05 and 5.15 and does not appear to be a glycoprotein. P2O is optimally stable at pH 8.0 and up to 60 °C. It is active over a broad pH range (5.0–9.0) with maximum activity at pH 8.0–8.5 and at 55 °C, and a broad substrate specificity. d-Glucose is the preferred substrate, but 1-β-aurothioglucose, 6-deoxy-d-glucose, l-sorbose, d-xylose, 5-thioglucose, d-glucono-1,5-lactone, maltose and 2-deoxy-d-glucose are also oxidised at relatively high rates. A Ping Pong Bi Bi mechanism was demonstrated for the P2O reaction at pH 8.0, with a catalytic constant (k cat) of 111.0 s−1 and an affinity constant (K m) of 1.43 mM for d-glucose and 83.2 μM for oxygen. Whereas the steady-state kinetics for glucose oxidation were unaffected by the medium at pH ≥ 7.0, at low pH both pH and buffer composition affected the P2O kinetics with the k cat/K m value decreasing with decreasing pH. The greatest effect was observed in acetate buffer (0.1 M, pH 4.5), where the k cat decreased to 60.9 s−1 and the K m increased to 240 mM. The activity of P2O was completely inhibited by 10 mM HgCl2, AgNO3 and ZnCl2, and 50% by lead acetate, CuCl2 and MnCl2.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 28 August 1996 / Received revision: 25 November 1996 / Accepted: 29 November 1996
Rights and permissions
About this article
Cite this article
Artolozaga, M., Kubátová, E., Volc, J. et al. Pyranose 2-oxidase from Phanerochaete chrysosporium– further biochemical characterisation. Appl Microbiol Biotechnol 47, 508–514 (1997). https://doi.org/10.1007/s002530050964
Issue Date:
DOI: https://doi.org/10.1007/s002530050964