Abstract
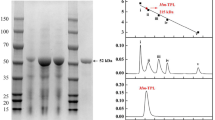
In this paper we report on the enzymatic preparation of d-p-trimethylsilylphenylalanine (d-TMS-Phe). First, dl-5-(p-trimethylsilylphenylmethyl)hydantoin␣(dl-TMS-Phe-Hyd) was synthesized chemically and subjected to bacterial hydrolysis to obtain N-carbamoyl-d-p-trimethylsilylphenylalanine (C-d-TMS-Phe), but no strains examined showed sufficient hydantoinase activity on this compound. However, Blastobacter sp. A17p-4, which is known to produce N-carbamoyl-d-amino acid amidohydrolase (DCase), was found to be able to hydrolyze C-dl-TMS-Phe prepared chemically from the hydantoin. When C-dl-TMS-Phe was hydrolyzed with cells of Blastobacter sp. A17p-4, its optical purity was low because N-carbamoyl-l-amino acid amidohydrolase (LCase) coexisted in the cells. DCase and LCase in the cell-free extract of Blastobacter sp. A17p-4 could be separated by DEAE-Sephacel column chromatography. The optimum pH for the hydrolysis of C-dl-TMS-Phe by the partially purified DCase was 8.0 and addition of 2.5 % N,N-dimethylformamide was effective in raising the substrate concentration without inactivation of DCase. Under the optimized conditions, highly optically pure (98 % enantiomeric excess) d-TMS-Phe could be obtained from C-dl-TMS-Phe with partially purified DCase.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 12 July 1996 / Received revision: 11 September 1996 / Accepted: 2 November 1996
Rights and permissions
About this article
Cite this article
Tsuji, Y., Yamanaka, H., Fukui, T. et al. Enzymatic preparation of d-p -trimethylsilylphenylalanine. Appl Microbiol Biotechnol 47, 114–119 (1997). https://doi.org/10.1007/s002530050898
Issue Date:
DOI: https://doi.org/10.1007/s002530050898