Abstract
Biobased polymers derived from plant oils are sustainable alternatives to petro based polymers. In recent years, multienzyme cascades have been developed for the synthesis of biobased ω-aminocarboxylic acids, which serve as building blocks for polyamides. In this work, we have developed a novel enzyme cascade for the synthesis of 12-aminododeceneoic acid, a precursor for nylon-12, starting from linoleic acid. Seven bacterial ω-transaminases (ω-TAs) were cloned, expressed in Escherichia coli and successfully purified by affinity chromatography. Activity towards the oxylipin pathway intermediates hexanal and 12-oxododecenoic acid in their 9(Z) and 10(E) isoforms was demonstrated for all seven transaminases in a coupled photometric enzyme assay. The highest specific activities were obtained with ω-TA from Aquitalea denitrificans (TRAD), with 0.62 U mg−1 for 12-oxo-9(Z)-dodecenoic acid, 0.52 U mg−1 for 12-oxo-10(E)-dodecenoic acid and 1.17 U mg−1 for hexanal. A one-pot enzyme cascade was established with TRAD and papaya hydroperoxide lyase (HPLCP-N), reaching conversions of 59% according to LC-ELSD quantification. Starting from linoleic acid, up to 12% conversion to 12-aminododecenoic acid was achieved with a 3-enzyme cascade comprising soybean lipoxygenase (LOX-1), HPLCP-N and TRAD. Higher product concentrations were achieved by the consecutive addition of enzymes compared to simultaneous addition at the beginning.
Key points
• Seven ω-transaminases converted 12-oxododecenoic acid into its corresponding amine.
• A three-enzyme cascade with lipoxygenase, hydroperoxide lyase, and ω-transaminase was established for the first time.
• A one-pot transformation of linoleic acid to 12-aminododecenoic acid, a precursor of nylon-12 was achieved.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyamides are important industrial polymers. Among them, nylon-12 exhibits properties that are centered between short-chain aliphatic nylons (e.g. nylon-6) and high-molecular-weight polymers (e.g., polyethylene). Nylon-12, as a high-performance polymer with good heat, UV and chemical resistance, is manufactured by ring opening polymerization of ω-laurolactam (Ladkau et al. 2016). Laurolactam is synthesized in a complex reaction cascade starting from the trimerization of petro-derived butadiene followed by cyclododecane oxime synthesis and Beckmann rearrangement (Karau et al. 2015). An increasing demand exists to replace fossil resources with renewable materials, forcing a switch in polymer production. For polyamide precursors, engineered whole-cell biocatalysts were used for their synthesis from fatty acids in multistep enzyme cascades. For example, 11-aminoundecanoic acid, a precursor for nylon-11, was obtained from 12-hydroxystearic acid using alcohol dehydrogenase, Baeyer–Villiger monooxygenase, esterase, and ω-transaminase (ω-TA) (Song et al. 2014). Furthermore, a whole-cell biocatalyst was designed using the alkane monooxygenase AlkBGT from Pseudomonas putida GPo1 and the ω-TA CV2025 from Chromobacterium violaceum for the synthesis of 12-aminododecanoic acid methyl ester from methyl laurate (Schrewe et al. 2013). The cascade was further improved by implementation of an alanine regeneration system to supply sufficient amounts of cosubstrate, overexpression of the outer membrane protein AlkL for substrate uptake and implementation of alcohol dehydrogenase AlkJ for increased alcohol oxidation (Ladkau et al. 2016; Ge et al. 2020).
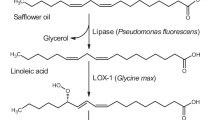
A disadvantage of the methyl laurate route towards nylon-12 is the utilization of tropical oils, which are the only source of natural lauric acid in large quantities. In contrast, linoleic acid is present in safflower or sunflower oil at high concentrations. In this work, we targeted a novel enzyme cascade for the synthesis of 12-aminododecenoic acid from linoleic acid (Fig. 1). For this purpose, lipoxygenase (LOX) and hydroperoxide lyase (HPL) originating from the oxylipin pathway were coupled to a transaminase reaction. In previous work from our group, 12-oxo-9(Z)-dodecenoic acid and hexanal were obtained from safflower oil in an enzyme cascade utilizing lipase, LOX, and HPL (Coenen et al. 2022). To date, the application of LOX and HPL has mainly focused on the green note synthesis of C6- and C9-aldehydes and their corresponding alcohols (Gigot et al. 2012; Vincenti et al. 2019; Stolterfoht et al. 2019). Otte et al. (2013) used 9-specific LOX and HPL for the synthesis of 9-oxononanoic acid. The integration of LOX and HPL into an E. coli host enabled the synthesis of the corresponding bifunctional azelaic acid employing endogenous oxidoreductases. The application of this intermediate as a polyester or polyamide building block was proposed (Otte et al. 2014).
Reaction scheme showing the transformation of linoleic acid with oxylipin pathway enzymes lipoxygenase and hydroperoxide lyase coupled to ω-transaminase. For the synthesis of hexylamine and 12-amino-9(Z)-dodecenoic acid. Dotted lines indicate the coupled photometrical enzyme assay with lactate dehydrogenase and NADH. Abbreviations are lipoxygenase (LOX), hydroperoxide lyase (HPL), ω-transaminase. (ω-TA), lactate dehydrogenase (LDH), and 13S-hydroperoxy-9(Z),11(E)-octadecadienoic acid (13S-HPODE)
ω-TA from C. violaceum has previously been shown to accept 12-oxododecanoic acid as a substrate (Schrewe et al. 2013), but the corresponding unsaturated 12-oxo-9(Z)-dodecenoic acid has not yet been tested as a ω-TA substrate. Therefore, we analyzed seven ω-TAs for their potential to synthesize 12-amino-9(Z)-dodecenoic acid. In addition to ω-TA from C. violaceum (TRCV) (Kaulmann et al. 2007), we chose enzymes from Paracoccus denitrificans (TRPD) (Rausch et al. 2013), and from uncultured bacteria most likely assigned to Acidihalobacter genus (TR2) and Rhodobacteraceae family (TR3 and TR6) (Coscolín et al. 2019). Additionally, new ω-TAs from Aquitalea denitrificans (TRAD) and Sulfitobacter delicatus (TRSD) were selected based on sequence homology analysis. The best-performing ω-TA was coupled in a one-pot reaction with lipoxygenase LOX-1 from Glycine max and hydroperoxide lyase HPLCP-N from Carica papaya to demonstrate the feasibility of linoleic acid-based synthesis of nylon-12 precursors.
Materials and methods
Reagents
The 12-aminododecanoic acid standard was purchased from Alfa Aesar (Haverhill, MA, USA), while the 12-oxo-9(Z)-dodecenoic acid and 12-oxo-10(E)-dodecenoic acid standards were purchased from Larodan (Solna, Sweden). Pyridoxal-5-phosphate monohydrate (PLP) was obtained from Acros Organics, Thermo Fisher Scientific (Waltham, MA, USA). Hexanal, hexylamine, soybean LOX-1, and L-lactate dehydrogenase (LDH) were supplied from Sigma Aldrich (St. Louis, MO, USA). Linoleic acid was obtained from Thermo Fisher Scientific (Waltham, MA, USA). β-Nicotine amide adenine dinucleotide disodium salt (NADH), isopropyl β-d-1-thiogalactopyranoside (IPTG), l-alanine, imidazole, ampicillin sodium salt, and kanamycin sulfate were purchased from Carl Roth (Karlsruhe, Germany). 13S-Hydroperoxy-9(Z),11(E)-octadecadienoic acid (13S-HPODE) was prepared from linoleic acid by a peroxidation reaction with LOX-1 from Glycine max as described previously (Gala Marti et al. 2021). All other chemicals were from Sigma-Aldrich (St. Louis, MO, USA), Thermo Fisher Scientific (Waltham, MA, USA) or Carl Roth (Karlsruhe, Germany).
Bioinformatic analysis
Putative novel transaminases homologous to ω-TA from C. violaceum CV2025 (TRCV: accession number WP_011135573.1) were identified with the Basic Local Alignment Search Tool (BLAST) (Altschul et al. 1990). We selected two new transaminases from A. denitrificans (TRAD: WP_159877958.1) and S. delicatus (TRSD; accession number WP_093738538.1) and compared them to the previously described transaminases from P. denitrificans (TRPD: accession number ABL72050.1), Acidihalobacter sp. (TR2: accession number MH588437), and uncultured Rhodobacteraceae bacteria (TR3: accession number MF158202 and TR6: accession number MF158205). A multiple sequence alignment of ω-TAs was performed with Clustal Omega (Sievers et al. 2011). A phylogenetic tree was constructed based on the neighbor-joining algorithm with a bootstrap value of 1000 and was generated with ClustalX (Thompson et al. 1997) and NJPlot (Perrière and Gouy 1996).
Cloning and expression of enzymes
The sequences coding for the transaminases TRAD, TRCV, TRPD, and TRSD were codon-optimized by BioCat GmbH (Heidelberg, Germany) for synthesis in E. coli. The optimized sequences trAD (accession number: OP866794), trCV (accession number: OP866795), trPD (accession number: OP866796), and trSD (accession number: OP866797) were cloned into the pET-21b( +) vector (Table 1). A sequence coding for a C-terminal hexahistidine tag (His6) was added for affinity purification. Chemically-competent E. coli BL21(DE3) cells were transformed with the respective vectors by heat shock at 42 °C for 90 s according to Hanahan (1983) and protein expression was carried out. Shaking flasks with baffles were inoculated with 2% (v/v) of an overnight culture, and cells were grown in terrific broth (TB) containing 100 µg ml−1 ampicillin at 37 °C until the OD600 reached 0.7–1. Then, cell expression was induced with 1 mM IPTG, and the temperature was lowered to 20 °C. Cells were cultured for 24 h before they were harvested by centrifugation at 4500 × g for 15 min at 4 °C. The supernatant was discarded, and the cell pellets were frozen at − 20 °C until further use.
The transaminases TR2, TR3, and TR6, which were cloned into pRhokHi-2 (for TR2) and pBXCH (for TR3 and TR6), were expressed in E. coli MC1061 as described previously (Coscolín et al. 2019). Hydroperoxide lyase from C. papaya (HPLCP-N) was expressed in E. coli BL21(DE3) using the pET-28a( +) expression vector as described previously (Coenen et al. 2022).
Protein purification
All transaminases were C-terminally His6-tagged and purified by metal affinity chromatography. For purification of TRAD, TRCV, TRPD, and TRSD, cell pellets from a 50-ml culture were suspended in 10 ml 50 mM potassium phosphate buffer pH 7.5 containing 500 mM NaCl and 40 mM imidazole. The suspensions were incubated on ice for 1 h before the cells were sonicated seven times for 15 s to obtain the crude extract (CE). The soluble fraction (SF) was separated after centrifugation of the CE for 45 min at 21,000 × g at 4 °C and loaded onto a HisTrap™ FF column (Cytiva, Marlborough, MA, USA). Nonspecifically bound proteins were removed by washing with 50 mM potassium phosphate buffer pH 7.5 with 500 mM NaCl and 40 mM imidazole, and the enzyme was eluted with 250 mM imidazole. The buffer was exchanged to 50 mM potassium phosphate pH 7.5 with 50 mM NaCl using a HiTrap® Desalting column (Cytiva, Marlborough, MA, USA). The eluted protein fractions were concentrated with Pierce™ Protein Concentrators PES, 10 K MWCO from Thermo Fisher Scientific (Waltham, MA, USA). The His6-tagged transaminases TR2, TR3, and TR6 were purified by affinity chromatography as described by Coscolín et al. (2019), and HPLCP-N was purified as described by Coenen et al. (2022). Protein concentrations were measured with Bradford reagent (Bradford 1976), and the purification process was monitored with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli 1970).
Preparation of 12-oxododecenoic acid
12-Oxo-9(Z)-dodecenoic acid was obtained from 13S-HPODE by a hydroperoxide lyase reaction using HPLCP-N. Reaction mixtures were prepared with 5 mM 13S-HPODE and 20 U ml−1 HPLCP-N in 50 mM potassium phosphate buffer pH 6 with 1 M NaCl for 15 min at 22 °C. The reaction products (12-oxo-9(Z)-dodecenoic acid and hexanal) were extracted by solvent extraction with methyl tert-butyl ether (MTBE). Due to the different volatilities of the substances, the solvent and hexanal could be evaporated with a vacuum concentrator, while 12-oxo-9(Z)-dodecenoic acid remained in the reaction vial. 12-Oxo-9(Z)-dodecenoic acid was dissolved in ethanol and frozen at − 80 °C until further use. The amount and purity of 12-oxo-9(Z)-dodecenoic acid were confirmed by GC analysis as described previously (Coenen et al. 2022). The remaining hexanal concentration was less than 1.5% compared to 12-oxo-9(Z)-dodecenoic acid.
Transaminase activity assay
Transaminase activity was measured in triplicate in a coupled photometric enzyme assay with lactate dehydrogenase (LDH) and NADH in a volume of 1 ml. Reactions were carried out in a cuvette containing 10 µl ω-TA solution, 10 µl of a 50 U ml−1 LDH solution (Sigma Aldrich, St. Louis, MO, USA), 0.1 mM substrate, 10 mM l-alanine and 0.1 mM pyridoxal-5-phosphate (PLP) in 50 mM potassium phosphate buffer pH 7.5 with 50 mM NaCl. 12-Oxo-9(Z)-dodecenoic acid, 12-oxo-10(E)-dodecenoic acid, or hexanal were used as substrates. Reactions were started by the addition of NADH to a concentration of 0.1 mM, and the decrease in absorbance was measured at 340 nm for 300 s at 22 °C. The extinction coefficient of NADH of 6220 M−1 cm−1 was used to determine the volumetric activity, and specific activities were calculated with Microsoft Excel based on the protein concentrations of the purified ω-TAs. Reactions without substrate addition were set up as negative control to confirm the assay. One unit is defined as the amount of enzyme that catalyzes the amination of 1 µmol of substrate (12-oxo-9(Z)-dodecenoic acid, 12-oxo-10(E)-dodecenoic acid, or hexanal) per minute with alanine as amine donor. The formation of NADH is proportional to the transformation of the acceptor substrates as outlined in Fig. 1.
Activity monitoring of the LOX, HPL, and ω-TA enzyme cascade
The product conversion in a one-pot reaction with HPLCP-N and ω-TA was analyzed photometrically as described above (paragraph 2.6). Additionally, 10 µl of a 20 U ml−1 HPLCP-N solution was added to the cuvette, and 0.1 mM 13S-HPODE was used as substrate. Reactions were started by the addition of NADH, and the decrease in the absorbance of NADH at 340 nm was monitored. Negative controls were performed by omitting the enzymes one by one to exclude a side reaction.
For a one-pot reaction with LOX-1, HPLCP-N, and ω-TA, the reaction mixtures were prepared in the same way. Additionally, 10 µl of 50 U ml−1 LOX-1 was added to the cuvette, and 0.1 mM linoleic acid was used as substrate. Again, reactions were started by the addition of NADH, and negative controls were performed by omitting the enzymes one by one.
Biocatalytic synthesis of 12-aminododecenoic acid and hexylamine
Transaminase reactions of 500 µl were set up with 5 U ml−1 of purified ω-TA (unit activity based on coupled photometric assay with 12-oxo-9(Z)-dodecenoic acid substrate), 50 mM l-alanine and 0.1 mM pyridoxal-5-phosphate in 50 mM potassium phosphate buffer pH 7.5 containing 50 mM NaCl. Optionally, DMSO was added at concentrations from 5 to 20%. Reactions were started by the addition of either 2.5 mM 12-oxo-9(Z)-dodecenoic acid, 12-oxo-10(E)-dodecenoic acid or hexanal. Reactions were typically carried out at 22 °C for 1 to 5 h. Then, 900 µl of a 50:50 acetonitrile–water mixture was added to 100 µl of reaction solution to stop the reaction, and the mixtures were used for HPLC analysis.
One-pot reactions with 20 U ml−1 HPLCP-N and 5 U ml−1 TRAD were carried out in a volume of 500 µl. Reactions were run at 22 °C using 50 mM l-alanine, 0.1 mM pyridoxal-5-phosphate, and 1 mM or 2.5 mM 13S-HPODE as substrate in 50 mM potassium phosphate buffer pH 7.5 containing 500 mM NaCl. The enzyme reactions were conducted either simultaneously for 1 h or consecutively, with HPLCP-N added at the beginning and incubated for 5 min before TRAD was added for 1 h. In a third approach, TRAD was added in the beginning and HPLCP-N was added consecutively every 10 min for 1 h.
In addition, one-pot reactions with LOX-1, HPLCP-N, and TRAD were conducted with 100 U ml−1 LOX-1, 20 U ml−1 HPLCP-N, and 5 U ml−1 TRAD in a total volume of 500 µl. The reactions were carried out as described above with 1 mM or 2.5 mM linoleic acid as substrate. Three different reaction settings were tested. Either all enzymes were added in the beginning and incubated for 4 h or LOX-1 was preincubated with linoleic acid for 3 h before HPLCP-N was added. After 5 min, TRAD was added together with l-alanine and pyridoxal-5-phosphate and reacted for another hour. In a third approach, LOX-1 was preincubated with linoleic acid for 3 h before TRAD was added to l-alanine and pyridoxal-5-phosphate, and HPLCP-N was added in portions every 10 min over a period of 1 h.
Analysis of products by liquid chromatography
Mass spectrometry data were obtained using an LC-30AD Nexera LC/MS system from Shimadzu (Kyoto, Japan) equipped with a Shimadzu SPD-M20A UV detector and Shimadzu LCMS-2020 mass spectrometry detector. Samples of 5 to 10 µl were loaded onto a Kromasil Orbit-100-C18 5 μm column (30 mm × 4.6 mm). Water (A) and acetonitrile (B) containing 0.1% formic acid were used as the mobile phase. A linear gradient was applied as follows: 0.1 min 20% B; 20% B to 90% B within 4 min; 1.1 min holding at 90% B. A flow rate of 1.0 ml min−1 was used. Samples were ionized by electron spray ionization (ESI) in negative and positive modes and recorded in the range of 50 to 700 m/z. For reference spectra, the standards 12-aminododecanoic acid, 12-oxo-9(Z)-dodecenoic acid, 12-oxo-10(E)-dodecenoic acid, hexanal, and hexylamine were used.
Quantification of 12-aminododecenoic acid was conducted using an LC-20AD XR Nexera Liquid Chromatograph from Shimadzu (Kyoto, Japan) equipped with an evaporative light scattering detector (ELSD) 100 (VWR, Radnor, PA, USA). Samples of 10 to 20 µl were loaded onto a LaChrom II + C18 RP column (250 mm × 4.6 mm, 5 µm particle size) from Hitachi (Chiyoda, Japan). As the mobile phase, water (A) and acetonitrile (B) containing 0.1% formic acid were used. A linear gradient was applied as follows: 20% B to 60% B within 14 min; 60% B to 80% B within 3 min; 80% B to 90% B within 5 min. A flow rate of 1.0 ml min−1 was used. Since 12-aminododecenoic acid is not commercially available, 12-aminododecanoic acid (Alfa Aesar, Haverhill, MA, USA) was used for calibration.
Results
Cloning and expression of ω-transaminases
ω-Transaminases (ω-TAs) have been successfully used for the amination of hydrophobic aldehydes including the synthesis of 12-aminododecanoic acid from the corresponding 12-oxododecanoic acid (Schrewe et al. 2013; Song et al. 2014). The structurally similar monounsaturated 12-oxododecenoic acid in its 9(Z)- or 10(E)-conformation can be obtained from linoleic acid by biocatalytic synthesis using the enzymes LOX and HPL with hexanal as a byproduct (Coenen et al. 2022). To elucidate whether these oxododecenoic acids can be used as substrates by ω-TAs, seven enzyme candidates were evaluated. As a starting point, we selected the transaminase from C. violaceum (TRCV) with proven activity towards 12-oxododecanoic acid. In addition, we chose the transaminase from P. denitrificans (TRPD) with a sequence identity of 38.31% to the C. violaceum enzyme (Table S1). This enzyme was reported to transform 6-oxohexanoic acid to the corresponding 6-aminohexanoic acid (Sattler et al. 2014). The transaminases TR2 from Acidihalobacter sp. as well as TR3 and TR6 from uncultured Rhodobacteraceae bacteria have been shown to convert bulky ketones and hexanal (Coscolín et al. 2019) and were also included in the enzyme screening. They share sequence identities of 58.94%, 34.54%, and 54.75%, respectively, to TRCV. In addition, a BLAST search was performed to identify additional homologues of TRCV. Transaminases from A. denitrificans (TRAD) and S. delicatus (TRSD) were selected with identities of 81.05% and 53.64% with respect to TRCV (Table S1).
Gene sequences encoding TRCV, TRAD, TRPD, and TRSD were codon-optimized for expression in E. coli and synthesized by BioCat GmbH (Heidelberg, Germany). They were cloned into the pET-21(b) + expression vector, and E coli BL21(DE3) was transformed with the respective vectors. The recombinant strains were grown in TB medium containing ampicillin at 20 °C for 24 h. Overexpression of the transaminases was analyzed with SDS-PAGE (Fig. 2a). Strong protein bands representing TRCV, TRAD, TRPD, and TRSD were visible in both the crude extracts (CE) and, after separation of cell fragments, in the soluble fractions (SF). All transaminases were His6-labelled and successfully purified by metal affinity chromatography (Fig. 2b). Proteins were eluted, and minor impurities were observed only in the TRPD and TRSD fractions. Overexpression of TRCV and TRAD resulted in total yields of 41 mg for TRAD and 80 mg for TRCV were obtained from 50 ml of culture broth. For TRPD and TRSD, the yields were lower; nevertheless, 13 and 17 mg of purified enzymes were obtained from 50 ml culture. Transaminases TR2, TR3, and TR6 were expressed in E. coli MC1061 as described previously (Coscolín et al. 2019). The protein masses of 45–55 kDa observed in SDS‒PAGE corresponded well with the calculated masses of the His6-tagged ω-TAs, which ranged from 48.42 to 51.25 kDa. The highest protein concentrations were measured in the eluate fractions of TRCV at 13.9 mg ml−1, followed by TR3 and TRAD at 5.21 mg ml−1 and 5.14 mg ml−1, respectively.
Purification and characterization of transaminases. a SDS-PAGE analysis after overexpression of transaminases TRAD, TRCV, TRPD, and TRSD with CE = crude extract, SF = soluble fraction and M = marker protein ladder. b SDS-PAGE of eluate fractions of transaminases after purification with metal affinity chromatography. c Specific activities of the purified ω-TAs with hexanal as substrate in U mg−.1 with 1 Unit (U)
Functional expression of the ω-TAs was analyzed with hexanal as a reference substrate (Fig. 2c). For this purpose, a coupled enzymatic activity assay was established with l-alanine, lactate dehydrogenase, and NADH (Fig. 1). The specific transaminase activities were calculated from the initial decrease in NADH. Negative controls in which enzymes, substrate or cofactor were sequentially omitted showed only minor background reactions, thus confirming the functionality of the enzyme assay. Figure 2c shows that all enzymes were functionally expressed and accepted hexanal as a substrate, with TRAD exhibiting the highest specific activity with 1.17 U mg−1, followed by TR6 and TRSD.
Transaminase activity analysis in a coupled enzyme system
12-Oxo-9(Z)-dodecenoic acid and 12-oxo-10(E)-dodecenoic acid were tested as ω-TA substrates in the coupled photometric assay. All seven enzymes accepted both oxoacids, revealing a broad substrate spectrum of the ω-TAs (Fig. 3a). In general, the shorter-chain aldehyde hexanal (Fig. 2c) was the best substrate for the ω-TAs except for TRPD, which exhibited higher specific activity for 12-oxo-9(Z)-dodecenoic acid. TRAD showed the highest specific activities, with 0.62 U mg−1 for 12-oxo-9(Z)-dodecenoic acid and 0.52 U mg−1 for 12-oxo-10(E)-dodecenoic acid. Although the double bonds are in direct proximity to the oxo-group, their position does not seem to affect their acceptance as transaminase substrates. Remarkably, five of the seven transaminases transformed the 10(E)- and 9(Z)-isomers with comparable activity. Especially in the 10(E)-configuration, a stabilized double bond system is formed in conjugation with the oxo-group, which should be a demanding substrate for transaminases. However, only TRPD and TR6 exhibited significantly lower activities towards the 10(E) isomer.
Substrates conversion of transaminases. a Specific activity of ω-TAs against different aldehyde substrates determined with a coupled photometrical enzyme assay with lactate dehydrogenase (LDH) and NADH at 340 nm. Blue: 12-oxo-9(Z)-dodecenoic acid and red: 12-oxo-10(E)-dodecenoic acid. b Conversion rate of NADH to NAD+ catalyzed by an enzyme cascade containing the ω-TAs in combination with HPLCP-N (blue bars) and in combination with HPLCP-N and LOX-1 (red bars) in a coupled photometrical enzyme assay with LDH and NADH
Coupling of transaminases with enzymes from the oxylipin pathway reveals a route for the synthesis of polyamide precursors from linoleic acid. To elucidate the feasibility of this pathway, we first combined ω-TAs with HPLCP-N and monitored the NADH decrease in the LDH coupled photometric assay. The initial overall activity could be monitored as the sole parameter because hexanal and oxododecenoic acid are measured simultaneously. 13S-HPODE, which was synthesized with LOX-1 as described previously (Gala Marti et al. 2021), was used as substrate, and purified papaya HPLCP-N expressed in E. coli (Coenen et al. 2022) was applied in the enzyme assay. Enzymatic activity was demonstrated for all seven ω-TAs (Fig. 3b). The highest conversion rates of 31.6 nmol min−1 ml−1 were obtained with TRCV, followed by TRAD and TR2. TRAD and TRCV already showed relatively high specific activities in the single substrate measurements (Fig. 3a), whereas TR2 exhibited relatively low specific activities. In contrast, TRSD performed better in the single-substrate measurements than in the enzyme cascade with HPLCP-N. Transaminase deactivation by the reactive hydroperoxide substrate 13S-HPODE or differences in transaminase substrate affinities may explain these differences.
Next, one-pot enzyme reactions were performed using soybean LOX-1, HPLCP-N and ω-TAs with linoleic acid as the substrate. Again, coupling to LDH and monitoring of NADH decrease was used for analysis of initial activities. A positive reaction was confirmed with all transaminases, although the overall activity was lower than that in the HPL–ω-TA system (Fig. 3b). The highest activity of 5.6 nmol min−1 ml−1 was measured with TRAD, followed by TRCV and TR2 exhibiting 4.9 nmol min−1 ml−1 each.
Development of an enzyme cascade with transaminase TRAD
TRAD was selected for the further development of a coupled enzyme cascade with oxylipin pathway enzymes. To monitor the synthesis of 12-aminododecenoic acid and hexylamine individually, the establishment of an analytical method was needed. For the detection of amino dodecenoic acids, we developed an LC-based analysis using ELSD detection (Fig. 4). Quantification of 12-aminododecenoic acid was possible using commercially available 12-aminododecanoic acid as a reference standard for the ELSD detector (Figure S1a). The formation of 12-aminododecenoic acid with TRAD was monitored over a period of 5 h. A maximum conversion of 47% was obtained at a substrate concentration of 2.5 mM within 1 h (Fig. 4). Hence, a 1-h reaction time was chosen for the development of the enzyme cascade.
For product verification, LC was coupled with a mass spectrometer. The mass spectrum of 12-aminododecenoic acid reveals a major peak at 214 m/z, which correlates to the molecular weight of its protonated form (Fig. S1b). Comparative analysis of 12-aminododecanoic acid possessing two extra hydrogen atoms gave a mass spectrum with a major peak at 216 m/z (Figure S1c). Transamination of 12-oxo-9(Z)- and 12-oxo-10(E)-dodecenoic acid (Figure S2a) resulted in similar mass spectra with the same peak at 214 m/z in each case (Figure S1d). The transformation of hexanal to hexylamine was monitored accordingly, and the mass spectrum of the TRAD reaction product correlated with the mass spectrum of a hexylamine reference exhibiting a peak of 102 m/z for the protonated molecule (Figure S2 b, c).
TRAD exhibited a pH optimum of 7.5 with remaining activities of approximately 40% at pH 6 and 20% at pH 9 (Fig. 5a). While LOX-1 has its pH optimum at approximately pH 9, HPLCP-N performs best at pH 6 (Coenen et al. 2022). Since our previous study showed sufficient activity of LOX-1 and HPLCP-N in a one-pot reaction at pH 7.5, this pH was expected to be suited for the three-enzyme cascade. The optimum NaCl concentration of TRAD was 50 mM, and more than 60% activity was maintained at high salt concentrations of 500 mM (Fig. 5b). HPLCP-N requires high salt concentrations for optimal activity (Coenen et al. 2022); therefore, a salt concentration of 500 mM NaCl was chosen for the enzyme cascade. LOX-1 and HPLCP-N are temperature-sensitive enzymes, and thus, a low reaction temperature should be applied in the enzyme cascade reaction. The highest 12-aminododecenoic acid conversion by TRAD was achieved at room temperature (22 °C) (Fig. 5c) and was chosen for all further experiments. It has been reported that transaminases, including TR2, TR3, and TR6, exhibit higher activities in the presence of DMSO (Coscolín et al. 2019). In contrast, we did not observe an activating effect of DMSO on TRAD (Fig. 5d); thus, the enzyme cascade was established under solvent-free conditions.
Analysis of reaction conditions for TRAD with monitoring of a pH, b salt concentration, c reaction temperature and d addition of DMSO. a, b pH and salt optima were analyzed photometrically with LDH and NADH coupling using 12-oxo-9(Z)-dodecenoic acid as substrate. The highest relative activities were set to 100% (for pH 7.5 1.7 U ml−1 and for 50 mM NaCl 1.8 U ml−1). c, d Temperature and DMSO optima were analyzed in biocatalytic reactions with LC-ELSD quantification
During the development of an enzyme cascade for the transformation of safflower oil to oxododecenoic acid, we discovered that the consecutive addition of enzymes resulted in better substrate conversion (Coenen et al. 2022). Therefore, simultaneous and consecutive enzyme addition was tested to determine the best conditions for a coupled HPLCP-N–TRAD reaction. Two modes of consecutive enzyme additions were analyzed. Either HPLCP-N was incubated with 13S-HPODE as substrate for 5 min before TRAD was added or TRAD was added at the beginning of the reaction, and HPLCP-N was dosed in 6 portions and added every 10 min for 1 h. The highest 12-aminododecenoic acid conversion of 59% was obtained with stepwise HPL addition at a substrate concentration of 1 mM (Fig. 6a). This setup prevents a too-rapid synthesis of 12-oxo-9(Z)-dodecenoic acid, which is unstable in the presence of high protein concentrations (Coenen et al. 2022). Thus, if 12-oxo-9(Z)-dodecenoic acid is formed stepwise, TRAD has enough time for the conversion to 12-aminododecenoic acid before 12-oxo-9(Z)-dodecenoic acid degradation. The conversion of 13S-HPODE with stepwise HPL addition was comparable to the reaction of 12-oxododecenoic acid at a substrate concentration of 2.5 mM with TRAD alone. Hence, this two-enzyme system performs equally well.
One-pot reactions of oxylipin pathway enzymes with ω-transaminase TRAD for the synthesis of 12-aminododecenoic acid. a HPLCP-N was coupled with TRAD using 1 mM and 2.5 mM 13S-HPODE as substrate. Blue bars = simultaneous enzyme addition, red bars = HPL addition before TRAD, grey bars = TRAD addition before HPL dosage in portions. b LOX-1 was coupled with HPLCP-N and TRAD using 1 mM and 2.5 mM linoleic acid as substrate. Blue bars = simultaneous enzyme addition, red bars = LOX-1 was added before HPLCP-N and then TRAD was added, grey bars = LOX-1 was added before TRAD addition and HPLCP-N dosage in portions
The three-enzyme cascade with LOX-1, HPLCP-N, and TRAD was analyzed in a similar way with both simultaneous and consecutive enzyme addition of HPL and ω-TA. According to the results of Gala Marti et al. (2021), the LOX-1 reaction proceeds over a period of 3–5 h under the chosen reaction conditions. Therefore, reactions were started with a 3-h preincubation with LOX-1 and linoleic acid as substrates. Then, HPLCP-N was applied 5 min before TRAD addition or TRAD was added first, and HPLCP-N was dosed in portions every 10 min for 1 h. The highest conversion of 12-aminododecenoic acid was again obtained in the reaction setup with stepwise HPL addition. The maximum conversion was lower than that in the 2-enzyme system and reached 12%; however, the general feasibility of a three-enzyme cascade with LOX-1, HPL and ω-TA was demonstrated (Fig. 6b).
Discussion
The vast majority of polymer production still relies on petroleum-based naphtha as a raw material. In recent years, several approaches have been developed for the synthesis of biologically based polymer precursors (Chung et al. 2015). 12-Aminododecanoic acid, the monomer for nylon-12 production, was synthesized from vegetable oil based raw materials with an engineered E. coli biocatalyst (Schrewe et al. 2013; Ladkau et al. 2016). However, this biocatalytic route uses lauric acid as the starting material, which can only be obtained in large quantities from palm kernel and coconut oil. Both coconut and oil palms can grow in wet tropical climates only, and an increase in oil production will increase pressure on pristine rainforests, leading to ecological problems (Dislich et al. 2017; Qaim et al. 2020). To circumvent this issue, we developed an alternative enzymatic route towards nylon-12 monomers, which uses linoleic acid as the starting material. Linoleic acid-rich oils are available from safflower and sunflower, and large-scale plantations are found in temperate and subtropical climate zones.
The ω-transaminases analyzed in our study originated from three different bacterial families: the Rhodobacteraceae family comprising TRPD, TRSD, TR3, and TR6, the Chromobacteriaceae family comprising TRCV and TRAD, and the Ectothiorhodospiraceae family comprising TR2. Differences between P. denitrificans and C. violaceum ω-TA become visible when analyzing the available crystal structures (Humble et al. 2012; Rausch et al. 2013). Interestingly, three residues in the active sites of the substrate pockets differ. While TRCV harbors residues M56, M166, and C418, TRPD harbors N53, K163, and L417 in the corresponding positions. These changes have been described to result in larger and more hydrophobic active sites in TRCV, which could lead, for example, in higher activity towards large, hydrophobic compounds such as long-chain aliphatic substrates (Rausch et al. 2013). In total, 17 amino acids were identified and designated to be important for substrate binding: four inside the S- (small) pocket and 12 inside the L- (large) pocket. Additionally, K285 (numeration of TRPD) was shown to bind the cofactor PLP. TRAD is the only transaminase that shares all 17 amino acid residues with TRCV (Figure S3), suggesting a similar structure of the substrate pockets. In contrast, the other ω-TAs share only 14 (TRSD, TR2, and TR6, TRPD) or 10 (TR3) of these amino acids, potentially leading to some structural variations. In accordance, phylogenetic analysis revealed that TRAD is most closely related to TRCV, whereas TRSD, TR2, and TR6 are significantly more distant, with sequence identities of approximately 55% (Figure S4, Table S1). TRPD and TR3 are the least homologous transaminases, with sequence identities of approximately 35% to TRCV and only 32% between each other.
Interestingly, despite differences in the sequences and amino acid architecture of their substrate pockets, all ω-TAs accepted unsaturated long-chain oxoacids as substrates. A clear substrate preference could not be deduced from the comparison of the three substrates tested. Most ω-TAs preferred hexanal, indicating that unsaturated oxoacids are more structurally challenging transaminase substrates. The only exception was TRPD, which showed higher specific activity for 12-oxo-9(Z)-dodecenoic acid than for hexanal. Additionally, the enzyme had a significantly lower activity with the 10(E) isomer, whereas the other transaminases showed less differentiation between the two isomers. In the coupled enzyme assay with HPLCP-N, a clearer distinction between the ω-TA groups was possible. Here, TRCV and TRAD showed the highest initial activities. Hexanal and ω-oxododecenoic acid are formed in situ by HPLCP-N, and thus, their substrate concentration is initially low. Therefore, TRCV and TRAD must possess higher substrate affinities than the other transaminases. The more hydrophobic active sites of these two transaminases may allow better substrate binding, making these enzymes better suited for the development of an enzyme cascade.
Coupling TRAD with HPLCP-N resulted in a similar substrate conversion as using TRAD alone. Thus, at substrate concentrations of 2.5 mM, the functionality of the two-enzyme cascade was proven. However, the three-enzyme cascade with LOX-1 resulted in a much lower conversion. In our previous study, we showed that the HPL reaction is significantly faster than the LOX reaction (Coenen et al. 2022). Hence, the slow formation of 13S-HPODE could be the cause of the lower overall activity observed in the coupled three-enzyme system. To circumvent this problem, reaction setups were tested, in which LOX-1 and linoleic acid were preincubated for 3 h before TRAD was added, and HPLCP-N was added in portions over an hour. Indeed, this reaction setup gave higher product concentrations than the simultaneous enzyme addition. Nevertheless, product concentrations of 12% and 3.5% at substrate concentrations of 1 mM and 2.5 mM, respectively, demand further reaction optimization. Low yields in a coupled LOX and HPL system have also been observed previously for the synthesis of 9-oxononanoic acid (Otte et al. 2013). Here, yields decreased significantly with increasing substrate concentration, and in accordance with our findings, the product conversion was significantly higher when LOX was preincubated with the substrate prior to the addition of HPL.
Another issue in the development of a LOX–HPL–ω-TA enzyme cascade is the low stability of 12-oxo-9(Z)-dodecenoic acid. Rapid isomerization to 12-oxo-10(E)-dodecenoic acid was observed within few minutes and was presumed to be caused either by keto-enol tautomerism or by Schiff base formation in the presence of high protein concentrations (Coenen et al. 2022). The development of a whole-cell biocatalyst for the continuous production of 12-aminododecenoic acid may overcome the aforementioned problems by adjusting the respective enzyme activities. Whole-cell biocatalysts have already been established with LOX and HPL (Buchhaupt et al. 2012; Otte et al. 2014); hence, incorporation of ω-TA should be feasible. In addition, a regeneration system for l-alanine and PLP could be engineered, which may be important for the conversion of higher substrate concentrations. In particular, an imbalance in alanine–pyruvate concentration can lead to an undesired shift of ω-TA-catalyzed alanine formation. Ge et al. (2020) established a whole-cell biocatalyst for the synthesis of 12-aminododecanoic acid from dodecanoic acid by enzymatic ω-hydroxylation, oxidation, and ω-amination in combination with a regeneration system for the cosubstrate l-alanine and the cofactors NADPH, NADH, and PLP and achieved a yield of 96.5% at a concentration of 5 mM. For cofactor regeneration, the endogenous cell pathways were used in combination with exogenous ribose 5-phosphate (R5P)-dependent PLP synthesis. A similar enzyme cascade with ω-TA and cofactor regeneration was developed by Kim et al. (2020), yielding up to 77.3% 11-aminoundecanoic acid from ricinoleic acid at substrate concentrations of 300 mM. These experiments indicate that an engineered whole-cell biocatalyst with a cofactor regeneration system may be suitable for a coupled LOX–HPL–ω-TA enzyme cascade.
It has to be mentioned that polymerization of our reaction product 12-amino-9(Z)-dodecenoic acid will result in unsaturated polyamide 12. Unsaturated polyamides were reported more than 80 years ago and the double bond is useful for polymer crosslinking (Pryde 1979). Several biobased unsaturated polyamides have been described more recently and were proposed for example as thermoreactive sealants (Radzik et al. 2020). To obtain saturated polyamide 12 hydrogenation of the double bond is needed. Standard chemical methods like hydrogenation with molecular hydrogen using palladium on coal catalysts may be applied (Mudiyanselage et al. 2014). Alternatively, ene-reductases may be an interesting biocatalytic option for double bond hydrogenation especially for the design of a whole cell biocatalyst. Ene-reductases exhibit broad substrate spectra and are currently applied for the asymmetric reduction of C–C double bonds (Toogood and Scrutton 2018).
In summary, seven ω-TAs were successfully cloned, expressed and purified, and their activity towards long-chain aldehydes was verified. TRAD from A. denitrificans was selected for the development of a three-enzyme cascade with soybean LOX-1 and papaya HPLCP-N. Starting from safflower oil-based linoleic acid, the synthesis of 12-aminododecenoic acid, a precursor for the synthesis of the biobased polyamide nylon-12, was demonstrated.
Data availability
Data are available on request.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buchhaupt M, Guder JC, Etschmann MMW, Schrader J (2012) Synthesis of green note aroma compounds by biotransformation of fatty acids using yeast cells coexpressing lipoxygenase and hydroperoxide lyase. Appl Microbiol Biotechnol 93:159–168. https://doi.org/10.1007/s00253-011-3482-1
Casadaban MJ, Cohen SN (1980) Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol 138:179–207. https://doi.org/10.1016/0022-2836(80)90283-1
Chung H, Yang JE, Ha JY, Chae TU, Shin JH, Gustavsson M, Lee SY (2015) Bio-based production of monomers and polymers by metabolically engineered microorganisms. Curr Opin Biotechnol 36:73–84. https://doi.org/10.1016/j.copbio.2015.07.003
Coenen A, Gala Marti V, Müller K, Sheremetiev M, Finamore L, Schörken U (2022) Synthesis of polymer precursor 12-oxododecenoic acid utilizing recombinant papaya hydroperoxide lyase in an enzyme cascade. Appl Biochem Biotechnol 194:6194–6212. https://doi.org/10.1007/S12010-022-04095-0/FIGURES/7
Coscolín C, Katzke N, García-Moyano A, Navarro-Fernández J, Almendral D, Martínez-Martínez M, Bollinger A, Bargiela R, Gertler C, Chernikova TN, Rojo D, Barbas C, Tran H, Golyshina O V., Koch R, Yakimov MM, Bjerga GEK, Golyshin PN, Jaeger KE, Ferrer M (2019) Bioprospecting reveals class III ω-transaminases converting bulky ketones and environmentally relevant polyamines. Appl Environ Microbiol 85. https://doi.org/10.1128/AEM.02404-18
Dislich C, Keyel AC, Salecker J, Kisel Y, Meyer KM, Auliya M, Barnes AD, Corre MD, Darras K, Faust H, Hess B, Klasen S, Knohl A, Kreft H, Meijide A, Nurdiansyah F, Otten F, Pe’er G, Steinebach S, Tarigan S, Tölle MH, Tscharntke T, Wiegand K (2017) A review of the ecosystem functions in oil palm plantations, using forests as a reference system. Biol Rev 92:1539–1569. https://doi.org/10.1111/BRV.12295
Gala Marti V, Coenen A, Schörken U (2021) Synthesis of linoleic acid 13-hydroperoxides from safflower oil utilizing lipoxygenase in a coupled enzyme system with in-situ oxygen generation. Catalysts 11:1119. https://doi.org/10.3390/catal11091119
Ge J, Yang X, Yu H, Ye L (2020) High-yield whole cell biosynthesis of Nylon 12 monomer with self-sufficient supply of multiple cofactors. Metab Eng 62:172–185. https://doi.org/10.1016/j.ymben.2020.09.006
Gigot C, Ongena M, Fauconnier ML, Muhovski Y, Wathelet JP, Du Jardin P, Thonart P (2012) Optimization and scaling up of a biotechnological synthesis of natural green leaf volatiles using Beta vulgaris hydroperoxide lyase. Process Biochem 47:2547–2551. https://doi.org/10.1016/j.procbio.2012.07.018
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580. https://doi.org/10.1016/S0022-2836(83)80284-8
Humble MS, Cassimjee KE, Hãkansson M, Kimbung YR, Walse B, Abedi V, Federsel HJ, Berglund P, Logan DT (2012) Crystal structures of the Chromobacterium violaceum ω-transaminase reveal major structural rearrangements upon binding of coenzyme PLP. FEBS J 279:779–792. https://doi.org/10.1111/J.1742-4658.2012.08468.X
Karau A, Sieber V, Haas T, Haeger H, Grammann K, Buehler B, Blank L, Schmid A, Jach G, Lalla B, Mueller A, Schullehner K, Welters P, Eggert T, Weckbecker A (2015) ω-Aminocarboxylic acids, ω-aminocarboxylic acid esters, or recombinant cells which produce lactams thereof. U.S. Patent No. 9,012,227
Kaulmann U, Smithies K, Smith MEB, Hailes HC, Ward JM (2007) Substrate spectrum of ω-transaminase from Chromobacterium violaceum DSM30191 and its potential for biocatalysis. Enzyme Microb Technol 41:628–637. https://doi.org/10.1016/j.enzmictec.2007.05.011
Kim TH, Kang SH, Han JE, Seo EJ, Jeon EY, Choi GE, Park JB, Oh DK (2020) Multilayer engineering of enzyme cascade catalysis for one-pot preparation of nylon monomers from renewable fatty acids. ACS Catal 10:4871–4878. https://doi.org/10.1021/acscatal.9b05426
Ladkau N, Assmann M, Schrewe M, Julsing MK, Schmid A, Bühler B (2016) Efficient production of the nylon 12 monomer ω-aminododecanoic acid methyl ester from renewable dodecanoic acid methyl ester with engineered Escherichia coli. Metab Eng 36:1–9. https://doi.org/10.1016/j.ymben.2016.02.011
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Mudiyanselage AY, Viamajala S, Varanasi S, Yamamoto K (2014) Simple ring-closing metathesis approach for synthesis of PA11, 12, and 13 precursors from oleic acid. ACS Sustain Chem Eng 2:2831–2836. https://doi.org/10.1021/SC500599U/SUPPL_FILE/SC500599U_SI_001.PDF
Otte KB, Kirtz M, Nestl BM, Hauer B (2013) Synthesis of 9-oxononanoic acid, a precursor for biopolymers. Chemsuschem 6:2149–2156. https://doi.org/10.1002/cssc.201300183
Otte KB, Kittelberger J, Kirtz M, Nestl BM, Hauer B (2014) Whole-cell one-pot biosynthesis of azelaic acid. ChemCatChem 6:1003–1009. https://doi.org/10.1002/cctc.201300787
Perrière G, Gouy M (1996) WWW-query: An on-line retrieval system for biological sequence banks. Biochimie 78:364–369. https://doi.org/10.1016/0300-9084(96)84768-7
Pryde EH (1979) Unsaturated Polyamides. J Macromol Sci Part C 17:1–35. https://doi.org/10.1080/00222357908080903
Qaim M, Sibhatu KT, Siregar H, Grass I (2020) Environmental, economic, and social consequences of the oil palm boom. Annu Rev Resour Econ 12:321–344. https://doi.org/10.1146/annurev-resource-110119-024922
Radzik P, Leszczyńska A, Pielichowski K (2020) Modern biopolyamide-based materials: synthesis and modification. Polym Bull 77:501–528. https://doi.org/10.1007/S00289-019-02718-X/FIGURES/7
Rausch C, Lerchner A, Schiefner A, Skerra A (2013) Crystal structure of the ω-aminotransferase from Paracoccus denitrificans and its phylogenetic relationship with other class III amino- transferases that have biotechnological potential. Proteins Struct Funct Bioinforma 81:774–787. https://doi.org/10.1002/prot.24233
Sattler JH, Fuchs M, Mutti FG, Grischek B, Engel P, Pfeffer J, Woodley JM, Kroutil W (2014) Introducing an in situ capping strategy in systems biocatalysis to access 6-aminohexanoic acid. Angew Chemie 126:14377–14381. https://doi.org/10.1002/ange.201409227
Schrewe M, Ladkau N, Bühler B, Schmid A (2013) Direct terminal alkylamino-functionalization via multistep biocatalysis in one recombinant whole-cell catalyst. Adv Synth Catal 355:1693–1697. https://doi.org/10.1002/adsc.201200958
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/MSB.2011.75
Song J-W, Lee J-H, Bornscheuer UT, Park J-B (2014) Microbial synthesis of medium-chain α, ω-dicarboxylic acids and ω-aminocarboxylic acids from renewable long-chain fatty acids. Adv Synth Catal 356:1782–1788. https://doi.org/10.1002/adsc.201300784
Stolterfoht H, Rinnofner C, Winkler M, Pichler H (2019) Recombinant lipoxygenases and hydroperoxide lyases for the synthesis of green leaf volatiles. J Agric Food Chem 67:13367–13392. https://doi.org/10.1021/acs.jafc.9b02690
Studier FW, Moffatt BA (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189:113–130. https://doi.org/10.1016/0022-2836(86)90385-2
Thompson J, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882. https://doi.org/10.1093/nar/25.24.4876
Toogood HS, Scrutton NS (2018) Discovery, characterization, engineering, and applications of ene-reductases for industrial biocatalysis. ACS Catal 8:3532–3549. https://doi.org/10.1021/ACSCATAL.8B00624/ASSET/IMAGES/LARGE/CS-2018-00624T_0013.JPEG
Vincenti S, Mariani M, Alberti J-C, Jacopini S, Brunini-Bronzini de Caraffa V, Berti L, Maury J (2019) Biocatalytic synthesis of natural green leaf volatiles using the lipoxygenase metabolic pathway. Catalysts 9:873. https://doi.org/10.3390/catal9100873
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by “Bundesministerium für Bildung und Forschung” (BMBF), FKZ 031B0671. MF acknowledges the financial support under Grants PID2020-112758RB-I00 and PDC2021-121534-I00 from the Ministerio de Ciencia e Innovación, Agencia Estatal de Investigación (AEI) (Digital Object Identifier https://doi.org/10.13039/501100011033), Fondo Europeo de Desarrollo Regional (FEDER) and the European Union (“NextGenerationEU/PRTR”).
Author information
Authors and Affiliations
Contributions
AC and US conceived the study. AC conducted experiments and MF and KEJ provided the TR2, TR3, and TR6 protein materials. US and MF were responsible for funding acquisition and KEJ and US supervised the project. AC and US wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
All authors declared their consent to participate.
Consent for publication
All authors declare their consent to publish their work.
Competing interests
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Coenen, A., Ferrer, M., Jaeger, KE. et al. Synthesis of 12-aminododecenoic acid by coupling transaminase to oxylipin pathway enzymes. Appl Microbiol Biotechnol 107, 2209–2221 (2023). https://doi.org/10.1007/s00253-023-12422-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12422-6