Abstract
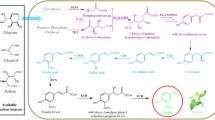
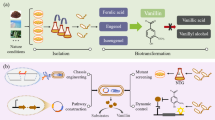
Vanillin is one of the most commonly used natural-occurring flavors in the world. This study successfully constructed an efficient whole-cell catalytic system for vanillin biosynthesis from ferulic acid by regulating feruloyl-CoA synthetase (FCS) and enoyl-CoA hydratase (ECH). First, we constructed an efficient cell-free catalytic system with FCS-Str (fcs from Streptomyces sp. V-1) and ECH-Str (ech from Streptomyces sp. V-1) combination at 1:1. The efficient cell-free catalytic system provided necessary strategies for optimizing the whole-cell catalytic system. Then, we constructed the recombinant Escherichia coli by heterologously expressing the fcs-Str and ech-Str combination. Moreover, E. coli JM109 was a better recombinant Escherichia coli than E. coli BL21 with higher vanillin production. Finally, we first adjusted the ratio of FCS and ECH in E. coli JM109 to 1:1 using two copies of fcs-Str. For higher vanillin production, we further optimized the induction conditions of E. coli JM109 to increase the amount of FCS and ECH. The optimized E. coli JM109-FE-F constructed in this study has the highest vanillin synthesis rate of converting 20 mM ferulic acid to 15 mM vanillin in 6 h among all of the E. coli catalytic systems. Our study made a significant contribution to the construction of the vanillin biosynthesis system and provided a valuable strategy for increasing vanillin production.
Key points
• The efficient cell-free vanillin biosynthesis system was constructed by FCS-Str and ECH-Str combination at 1:1.
• Escherichia coli JM109 was determined as a better recombinant Escherichia coli than E. coli BL21 with higher vanillin production.
• Escherichia coli JM109-FE-F with two copies of fcs-Str and one copy of ech-Str has the highest catalytic efficiency for vanillin production.






Similar content being viewed by others
Data availability
All datasets obtained for this study are included in the manuscript/supplementary material.
References
Arya SS, Rookes JE, Cahill DM, Lenka SK (2021) Vanillin: a review on the therapeutic prospects of a popular flavouring molecule. Adv Tradit Med 21:1–17
Banerjee G, Chattopadhyay P (2018) Vanillin biotechnology: the perspectives and future. J Sci Food Agr 99:499–506
Barghini P, Di Gioia D, Fava F, Ruzzi M (2007) Vanillin production using metabolically engineered Escherichia coli under non-growing conditions. Microb Cell Fact 6:13
Chakraborty D, Gupta G, Kaur B (2016) Metabolic engineering of E.coli top 10 for production of vanillin through FA catabolic pathway and bioprocess optimization using RSM. Protein Expres Purif 128:123–133
Chattopadhyay P, Banerjee G, Sen SK (2018) Cleaner production of vanillin through biotransformation of ferulic acid esters from agroresidue by Streptomyces sannanensis. J Clean Prod 182:272–279
Davis MM (2003) The problem of plain vanilla peptides. Nat Immunol 4:649–650
Di Gioia D, Luziatelli F, Negroni A, Ficca AG, Fava F, Ruzzi M (2011) Metabolic engineering of Pseudomonas fluorescens for the production of vanillin from ferulic acid. J Biotechnol 156:309–316
Fleige C, Meyer F, Steinbuchel A (2016) Metabolic engineering of the actinomycete Amycolatopsis sp. Strain ATCC 39116 towards enhanced production of natural vanillin. Appl Environ Microbiol 82:3410–3419
Furuya T, Miura M, Kuroiwa M, Kino K (2015) High-yield production of vanillin from ferulic acid by a coenzyme-independent decarboxylase/oxygenase two-stage process. New Biotechnol 32:335–339
Gallage NJ, Moller BL (2015) Vanillin-bioconversion and bioengineering of the most popular plant flavor and its de novo biosynthesis in the vanilla orchid. Mol Plant 8:40–57
Gallage NJ, Hansen EH, Kannangara R, Olsen CE, Motawia MS, Jorgensen K, Holme I, Hebelstrup K, Grisoni M, Moller BL (2014) Vanillin formation from ferulic acid in vanilla planifolia is catalysed by a single enzyme. Nat Commun 5:4037
Gallage NJ, Jorgensen K, Janfelt C, Nielsen AJZ, Naake T, Dunski E, Dalsten L, Grisoni M, Moller BL (2018) The intracellular localization of the vanillin biosynthetic machinery in pods of vanilla planifolia. Plant Cell Physiol 59:304–318
Hua D, Ma C, Song L, Lin S, Zhang Z, Deng Z, Xu P (2007) Enhanced vanillin production from ferulic acid using adsorbent resin. Appl Microbiol Biotechnol 74:783–790
Tamara K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10(3):512–26
Khoyratty S, Kodja H, Verpoorte R (2018) Vanilla flavor production methods: a review. Ind Crop Prod 125:433–442
Kunjapur AM, Prather KLJ (2019) Development of a vanillate biosensor for the vanillin biosynthesis pathway in E. coli. ACS Synth Biol 8:1958–1967
Lee EG, Yoon SH, Das A, Lee SH, Li C, Kim JY, Choi MS, Oh DK, Kim SW (2009) Directing vanillin production from ferulic acid by increased acetyl-CoA consumption in recombinant Escherichia coli. Biotechnol Bioeng 102:200–208
Li L, Liao Y, Luo Y, Zhang G, Liao X, Zhang W, Zheng S, Han S, Lin Y, Liang S (2019) Improved efficiency of the desulfurization of oil sulfur compounds in Escherichia coli using a combination of desensitization engineering and DszC overexpression. ACS Synth Biol 8:1441–1451
Luziatelli F, Brunetti L, Ficca AG, Ruzzi M (2019) Maximizing the efficiency of vanillin production by biocatalyst enhancement and process optimization. Front Bioeng Biotechnol 7:279
Marisch K, Bayer K, Scharl T, Mairhofer J, Krempl PM, Hummel K, Razzazi-Fazeli E, Striedner G (2013) A comparative analysis of industrial Escherichia coli K-12 and B strains in high-glucose batch cultivations on process-, transcriptome- and proteome level. PLoS One 8:e70516
Martu GA, Clinoiu LF, Vodnar DC (2021) Bio-vanillin: towards a sustainable industrial production. Trends Food Sci Tech 109:579–592
Meyer F, Pupkes H, Steinbuchel A (2017) Development of an improved system for the generation of knockout mutants of Amycolatopsis sp. strain ATCC 39116. Appl Environ Microbiol 83:e02660
Muheim A, Lerch K (1999) Towards a high-yield bioconversion of ferulic acid to vanillin. Appl Microbiol Biot 51:456–461
Ni J, Gao YY, Tao F, Liu HY, Xu P (2018) Temperature-directed biocatalysis for the sustainable production of aromatic aldehydes or alcohols. Angewandte Chemie-International Edition 57:1214–1217
Overhage J, Priefert H, Rabenhorst J, Steinbuchel A (1999a) Biotransformation of eugenol to vanillin by a mutant of Pseudomonas sp. strain HR199 constructed by disruption of the vanillin dehydrogenase (vdh) gene. Appl Microbiol Biotechnol 52:820–828
Overhage J, Priefert H, Steinbuchel A (1999b) Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl Environ Microbiol 65:4837–4847
Pattrick CA, Webb JP, Green J, Chaudhuri RR, Collins MO, Kelly DJ (2019) Proteomic profiling, transcription factor modeling, and genomics of evolved tolerant strains elucidate mechanisms of vanillin toxicity in Escherichia coli. mSystems 4:e00163-00119
Plaggenborg R, Overhage J, Steinbuchel A, Priefert H (2003) Functional analyses of genes involved in the metabolism of ferulic acid in Pseudomonas putida KT2440. Appl Microbiol Biotechnol 61:528–535
Priefert H, Rabenhorst J, Steinbüchel A (2001) Biotechnological production of vanillin. Appl Microbiol Biot 56:296–314
Ravi K, Garcia-Hidalgo J, Gorwa-Grauslund MF, Liden G (2017a) Conversion of lignin model compounds by Pseudomonas putida KT2440 and isolates from compost. Appl Microbiol Biot 101:5059–5070
Ravi K, García-Hidalgo J, Gorwa-Grauslund MF, Lidén G (2017b) Conversion of lignin model compounds by Pseudomonas putida KT2440 and isolates from compost. Appl Microbiol Biotechnol 101:5059–5070
Schwab W, Lange BM, Wüst M (2018) Vanilla: the most popular flavour. Biotechnology of Natural Products 1:3–24
Shan Q, Ping SA, Ying L, Zhi C, Yuan HC, Hong MY (2019) Elevated β-carotene synthesis by the engineered Rhodobactersphaeroides with enhanced CrtY expression. J Agr Food Chem 67:9560–9568
Sudhir K, Glen S, Michael L, Christina K, Koichiro T (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Yang W, Tang H, Ni J, Wu Q, Hua D, Tao F, Xu P (2013) Characterization of two Streptomyces enzymes that convert ferulic acid to vanillin. PLoS One 8:e67339
Yao J, He Y, Su N, Bharath SR, Tao Y, Jin JM, Chen W, Song H, Tang SY (2020) Developing a highly efficient hydroxytyrosol whole-cell catalyst by de-bottlenecking rate-limiting steps. Nat Commun 11:1515
Yeoh JW, Jayaraman SS, Tan SG, Jayaraman P, Holowko MB, Zhang J, Kang CW, Leo HL, Poh CL (2021) A model-driven approach towards rational microbial bioprocess optimization. Biotechnol Bioeng 118:305–318
Yoon SH, Han MJ, Jeong H, Lee CH, Xia XX, Lee DH, Shim JH, Lee SY, Oh TK, Kim JF (2012) Comparative multi-omics systems analysis of Escherichia coli strains B and K-12. Genome Biol 13:37
Yoon SH, Lee EG, Das A, Lee SH, Kim SW (2007) Enhanced vanillin production from recombinant E.coli using ntg mutagenesis and adsorbent resin. Biotechnol Progr 23:1143–1148
Yoon SH, Li C, Kim JE, Lee SH, Yoon JY, Choi MS, Seo WT, Yang JK, Kim JY, Kim SW (2005) Production of vanillin by metabolically engineered Escherichia coli. Biotechnol Lett 27:1829–1832
Yu L, Shi ZZ, Li CM (2015) Atom transfer radical polymerization to fabricate monodisperse poly[glycidyl methacrylate-co-poly (ethylene glycol) methacrylate] microspheres and its application for protein affinity purification. J Colloid Interface Sci 453:151–158
Zhao FL, Zhang C, Zhang C, Tang Y, Ye BC (2016) A genetically encoded biosensor for in vitro and in vivo detection of NADP. Biosens Bioelectron 77:901–906
Zhong XY, Lin JM, Zhou JH, Xu W, Hong ZF (2015) Anti-proliferative effects of qianliening capsules on prostatic hyperplasia in vitro and in vivo. Mol Med Rep 12:1699–1708
Funding
This research was supported by the 2020 Team Innovation Project from the Fundamental Scientific Research Special Capital Fund of the National Universities, China (100034/1301030160) and the Natural Science Foundation of China (NSFC) Project (grant number 32172145). Fundamental Research Funds for Central Universities of the Central South University,100034/1301030160,Yong Hong Meng,Natural Science Foundation of China (NSFC) Project,32172145,Yong Hong Meng
Author information
Authors and Affiliations
Contributions
QC, DX, SQ, and YM designed the experiments. QC performed the experiments. QC and YM were responsible for data processing and analysis. QC, YM, and CH wrote and revised manuscripts.
Corresponding author
Ethics declarations
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Q.H., Xie, D.T., Qiang, S. et al. Developing efficient vanillin biosynthesis system by regulating feruloyl-CoA synthetase and enoyl-CoA hydratase enzymes. Appl Microbiol Biotechnol 106, 247–259 (2022). https://doi.org/10.1007/s00253-021-11709-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11709-w