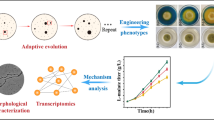
Abstract
Regulating morphology engineering and fermentation of Aspergillus oryzae makes it possible to increase the titer of L-malate. However, the existing L-malate-producing strain has limited L-malate production capacity and the fermentation process is insufficiently mature, which cannot meet the needs of industrial L-malate production. To further increase the L-malate production capacity of A. oryzae, we screened out a mutant strain (FMME-S-38) that produced 79.8 g/L L-malate in 250-mL shake flasks, using a newly developed screening system based on colony morphology on the plate. We further compared the extracellular nitrogen (N1) and intracellular nitrogen (N2) contents of the control and mutant strain (FMME-S-38) to determine the relationship between the curve of nitrogen content (N1 and N2) and the L-malate titer. This correlation was then used to optimize the conditions for developing a novel nitrogen supply strategy (initial tryptone concentration of 6.5 g/L and feeding with 3 g/L tryptone at 24 h). Fermentation in a 7.5-L fermentor under the optimized conditions further increased the titer and productivity of L-malate to 143.3 g/L and 1.19 g/L/h, respectively, corresponding to 164.9 g/L and 1.14 g/L/h in a 30-L fermentor. This nitrogen regulation-based strategy cannot only enhance industrial-scale L-malate production but also has generalizability and the potential to increase the production of similar metabolites.
Key Points
• Construction of a new screening system based on colony morphology on the plate.
• A novel nitrogen regulation strategy used to regulate the production of L-malate.
• A nitrogen supply strategy used to maximize the production of L-malate.





Similar content being viewed by others
Data availability
The data that support the figures within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Adrio JL, Demain AL (2006) Genetic improvement of processes yielding microbial products. FEMS Microbiol Rev 30(2):187–214. https://doi.org/10.1111/j.1574-6976.2005.00009.x
Battat E, Peleg Y, Bercovitz A, Rokem JS, Goldberg I (1991) Optimization of L-malic acid production by Aspergillus flavus in a stirred fermenter. Biotechnol Bioeng 37(11):1108–1116. https://doi.org/10.1002/bit.260371117
Brown SH, Bashkirova L, Berka R, Chandler T, Doty T, McCall K, McCulloch M, McFarland S, Thompson S, Yaver D, Berry A (2013) Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of L-malic acid. Appl Microbiol Biotechnol 97(20):8903–8912. https://doi.org/10.1007/s00253-013-5132-2
Cao WF, Luo JQ, Zhao J, Qiao CS, Ding LH, Qi BK, Su Y, Wan YH (2012) Intensification of beta-poly(L: -malic acid) production by Aureobasidium pullulans ipe-1 in the late exponential growth phase. J Ind Microbiol Biotechnol 39(7):1073-80. https://doi.org/10.1007/s10295-012-1111-3
Carsanba E, Papanikolaou S, Fickers P, Erten H (2019) Screening various Yarrowia lipolytica strains for citric acid production. Yeast 36(5):319-327. https://doi.org/10.1002/yea.3389
Chen XL, Wang YC, Dong XX, Hu GP, Liu LM (2017) Engineering rTCA pathway and C4-dicarboxylate transporter for L-malic acid production. Appl Microbiol Biotechnol 101(10):4041-4052. https://doi.org/10.1007/s00253-017-8141-8
Chen XL, Xu GQ, Xu N, Zou W, Zhu P, Liu LM, Chen J (2013) Metabolic engineering of Torulopsis glabrata for malate production. Metab Eng 19:10-16. https://doi.org/10.1016/j.ymben.2013.05.002
Chen XL, Zhou J, Ding Q, Luo QL, Liu LM (2019) Morphology engineering of Aspergillus oryzae for L-malate production. Biotechnol Bioeng 116(10):2662-2673. https://doi.org/10.1002/bit.27089
Chi Z, Wang ZP, Wang GY, Khan I, Chi ZM (2016) Microbial biosynthesis and secretion of L-malic acid and its applications. Crit Rev Biotechnol 36(1):99–107. https://doi.org/10.3109/07388551.2014.924474
Christensen T, Hynes MJ, Davis MA (1998) Role of the regulatory gene areA of Aspergillus oryzae in nitrogen metabolism. Appl Environ Microbiol 64(9):3232-3237.
Chu FF, Cheng J, Li K, Wang YG, Li X, Yang WJ (2020) Enhanced lipid accumulation through a regulated metabolic pathway of phosphorus luxury uptake in the microalga Chlorella Vulgaris under nitrogen starvation and phosphorus depletion. ACS Sustain Chem Eng 8(22):8137-8147. https://doi.org/10.1021/acssuschemeng.9b07447
Ding H, Inoue S, Ljubimov AV, Patil R, Portilla Arias J, Hu J, Konda B, Wawrowsky KA, Fujita M, Karabalin N, Sasaki T, Black KL, Holler E, Ljubimova JY (2010) Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release. Proc Natl Acad Sci U S A 107(42):18143-18148. https://doi.org/10.1073/pnas.1003919107
Ding Q, Luo QL, Zhou J, Chen XL, Liu LM (2018) Enhancing L-malate production of Aspergillus oryzae FMME218-37 by improving inorganic nitrogen utilization. Appl Microbiol Biotechnol 102(20):8739-8751. https://doi.org/10.1007/s00253-018-9272-2
Dong XX, Chen XL, Qian YY, Wang YC, Wang L, Qiao WH, Liu LM (2017) Metabolic engineering of Escherichia coli W3110 to produce L-malate. Biotechnol Bioeng 114(3):656-664. https://doi.org/10.1002/bit.26190
Downes DJ, Dayis MA, Kreutzberger SD, Taig BL, Todd RB (2013) Regulation of the NADP-glutamate dehydrogenase gene gdhA in Aspergillus nidulans by the Zn(II)2Cys6 transcription factor LeuB. Microbiology-Sgm 159(12):2467-2480. https://doi.org/10.1099/mic.0.071514-0
Gao C, Wang SH, Hu GP, Guo L, Chen XL, Xu P, Liu LM (2018) Engineering Escherichia coli for malate production by integrating modular pathway characterization with CRISPRi-guided multiplexed metabolic tuning. Biotechnol Bioeng 115(3):661-672. https://doi.org/10.1002/bit.26486
Gao YY, Yang MC, Wang CH (2013) Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 147:484-491. https://doi.org/10.1016/j.biortech.2013.08.066
Guo L, Zhang F, Zhang C, Hu GP, Gao C, Chen XL, Liu LM (2018) Enhancement of malate production through engineering of the periplasmic rTCA pathway in Escherichia coli. Biotechnol Bioeng 115(6):1571-1580. https://doi.org/10.1002/bit.26580
Harper M, Lee CJ (2012) Genome-wide analysis of mutagenesis bias and context sensitivity of N-methyl-N'-nitro-N-nitrosoguanidine (NTG). Mutat Res 731(1-2):64-7. https://doi.org/10.1016/j.mrfmmm.2011.10.011
Hu GP, Zhou J, Chen XL, Qian YY, Gao C, Guo L, Xu P, Chen W, Chen J, Li Y, Liu LM (2018) Engineering synergetic CO2-fixing pathways for malate production. Metab Eng 47:496-504. https://doi.org/10.1016/j.ymben.2018.05.007
Huang DM, Song YY, Liu YL, Qin Y (2019) A new strain of Aspergillus tubingensis for high-activity pectinase production. Braz J Microbiol 50(1):53-65. https://doi.org/10.1007/s42770-018-0032-3
Iyyappan J, Baskar G, Gnansounou E, Pandey A, Raaman JK, Bharathiraja B, Praveenkumar R (2019) Recent advances in microbial production of malic acid from renewable byproducts. Rev Environ Sci Bio-Technol 18(3):579-595. https://doi.org/10.1007/s11157-019-09503-2
Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO (2008) Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99(5):1140-53. https://doi.org/10.1002/bit.21694
Karahalil E, Coban HB, Turhan I (2019) A current approach to the control of filamentous fungal growth in media: microparticle enhanced cultivation technique. Crit Rev Biotechnol 39(2):192–201. https://doi.org/10.1080/07388551.2018.1531821
Khan I, Nazir K, Wang ZP, Liu GL, Chi ZM (2014) Calcium malate overproduction by Penicillium viticola 152 using the medium containing corn steep liquor. Appl Microbiol Biotechnol 98(4):1539-1546. https://doi.org/10.1007/s00253-013-5326-7
Knuf C, Nookaew I, Brown SH, McCulloch M, Berry A, Nielsen J (2013) Investigation of malic acid production in Aspergillus oryzae under nitrogen starvation conditions. Appl Environ Microbiol 79(19):6050-6058. https://doi.org/10.1128/aem.01445-13
Kövilein A, Kubisch C, Cai L, Ochsenreither K (2019) Malic acid production from renewables: a review. J Chem Technol Biotechnol 95(3):513–526. https://doi.org/10.1002/jctb.6269
Kowalski CH, Kerkaert JD, Liu KW, Bond MC, Hartmann R, Nadell CD, Stajich JE, Cramer RA (2019) Fungal biofilm morphology impacts hypoxia fitness and disease progression. Nat Microbiol 4(12):2430-2441. https://doi.org/10.1038/s41564-019-0558-7
Kuck U, Hoff B (2010) New tools for the genetic manipulation of filamentous fungi. Appl Microbiol Biotechnol 86(1):51-62. https://doi.org/10.1007/s00253-009-2416-7
Kuypers MMM, Marchant HK, Kartal B (2018) The microbial nitrogen-cycling network. Nat Rev Microbiol 16(5):263-276. https://doi.org/10.1038/nrmicro.2018.9
Lee JC, Park HR, Park DJ, Son KH, Yoon KH, Kim YB, Kim CJ (2003) Production of teicoplanin by a mutant of Actinoplanes teicomyceticus. Biotechnol Lett 25(7):537-540. https://doi.org/10.1023/a:1022842203917
Li XJ, Liu Y, Yang Y, Zhang H, Wang HL, Wu Y, Zhang M, Sun T, Cheng JS, Wu XF, Pan LJ, Jiang ST, Wu HW (2014) High levels of malic acid production by the bioconversion of corn straw hydrolyte using an isolated Rhizopus delemar strain. Biotechnol Bioprocess Eng 19(3):478-492. https://doi.org/10.1007/s12257-014-0047-z
Li XT, Li W, Zhai J, Wei HX (2018a) Effect of nitrogen limitation on biochemical composition and photosynthetic performance for fed-batch mixotrophic cultivation of microalga Spirulina platensis. Bioresour Technol 263:555-561. https://doi.org/10.1016/j.biortech.2018.05.046
Li Y, Hugenholtz J, Chen J, Lun SY (2002) Enhancement of pyruvate production by Torulopsis glabrata using a two-stage oxygen supply control strategy. Appl Microbiol Biotechnol 60(1-2):101-6. https://doi.org/10.1007/s00253-002-1064-y
Li ZJ, Hong PH, Da YY, Li LK, Stephanopoulos G (2018b) Metabolic engineering of Escherichia coli for the production of L-malate from xylose. Metab Eng 48:25-32. https://doi.org/10.1016/j.ymben.2018.05.010
Lim J, Choi YH, Hurh BS, Lee I (2019) Strain improvement of Aspergillus sojae for increased L-leucine aminopeptidase and protease production. Food Sci Biotechnol 28(1):121-128. https://doi.org/10.1007/s10068-018-0427-9
Ling XP, Guo J, Liu XT, Zhang X, Wang N, Lu YH, Ng IS (2015) Impact of carbon and nitrogen feeding strategy on high production of biomass and docosahexaenoic acid (DHA) by Schizochytrium sp. LU310. Bioresour Technol 184:139-147. https://doi.org/10.1016/j.biortech.2014.09.130
Liu JJ, Xie ZP, Shin H-D, Li JH, Du GC, Chen J, Liu L (2017) Rewiring the reductive tricarboxylic acid pathway and L-malate transport pathway of Aspergillus oryzae for overproduction of L-malate. J Biotechnol 253:1-9. https://doi.org/10.1016/j.jbiotec.2017.05.011
Liu XY, Yu XJ, Wang ZP, Xia J, Yan YB, Hu L, Wang XY, Xu JX, He AY, Zhao PS (2020) Enhanced erythritol production by a Snf1-deficient Yarrowia lipolytica strain under nitrogen-enriched fermentation condition. Food Bioprod Process 119:306-316. https://doi.org/10.1016/j.fbp.2019.11.012
Longacre A, Reimers JM, Gannon JE, Wright BE (1997) Flux analysis of glucose metabolism in Rhizopus oryzae for the purpose of increasing lactate yields. Fungal Genet Biol 21(1):30–39. https://doi.org/10.1006/fgbi.1996.0952
Ottenheim C, Werner KA, Zimmermann W, Wu JC (2015) Improved endoxylanase production and colony morphology of Aspergillus niger DSM 26641 by γ-ray induced mutagenesis. Biochem Eng J 94:9-14. https://doi.org/10.1016/j.bej.2014.10.020
Pan L, Chen XS, Liu MM, Liu YJ, Mao ZG (2017) Efficient production of epsilon-poly-L-lysine from glucose by two-stage fermentation using pH shock strategy. Process Biochem 63:8-15. https://doi.org/10.1016/j.procbio.2017.08.008
Papagianni M (2007) Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modeling. Biotechnol Adv 25(3):244-63. https://doi.org/10.1016/j.biotechadv.2007.01.002
Porcel EMR, Lopez JLC, Perez JAS, Sevilla JMF, Chisti Y (2005) Effects of pellet morphology on broth rheology in fermentations of Aspergillus terreus. Biochem Eng J 26(2-3):139-144. https://doi.org/10.1016/j.bej.2005.04.011
Rokem JS, Lantz AE, Nielsen J (2007) Systems biology of antibiotic production by microorganisms. Nat Prod Rep 24(6):1262-1287. https://doi.org/10.1039/b617765b
Sagnak R, Cochot S, Molina-Jouve C, Nicaud J-M, Guillouet SE (2018) Modulation of the glycerol phosphate availability led to concomitant reduction in the citric acid excretion and increase in lipid content and yield in Yarrowia lipolytica. J Biotechnol 265:40-45. https://doi.org/10.1016/j.jbiotec.2017.11.001
Sun XW, Wu HF, Zhao GH, Li ZM, Wu XH, Liu H, Zheng ZM (2018) Morphological regulation of Aspergillus niger to improve citric acid production by chsC gene silencing. Bioprocess Biosyst Eng 41(7):1029-1038. https://doi.org/10.1007/s00449-018-1932-1
Swift RJ, Craig SH, Wiebe MG, Robson GD, Trinci APJ (2000) Evolution of Aspergillus niger and A. nidulans in glucose-limited chemostat cultures, as indicated by oscillations in the frequency of cycloheximide resistant and morphological mutants. Mycol Res 104(3):333-337. https://doi.org/10.1017/S0953756299001136
Wakai S, Yoshie T, Asai Nakashima N, Yamada R, Ogino C, Tsutsumi H, Hata Y, Kondo A (2014) L-lactic acid production from starch by simultaneous saccharification and fermentation in a genetically engineered Aspergillus oryzae pure culture. Bioresour Technol 173:376-383. https://doi.org/10.1016/j.biortech.2014.09.094
Wang XYZ, Dong JJ, Xu GC, Han RZ, Ni Y (2016) Enhanced curdlan production with nitrogen feeding during polysaccharide synthesis by Rhizobium radiobacter. Carbohydr Polym 150:385-391. https://doi.org/10.1016/j.carbpol.2016.05.036
Xu YX, Zhou YT, Cao W, Liu H (2020) Improved production of malic acid in Aspergillus niger by abolishing citric acid accumulation and enhancing glycolytic flux. ACS Synth Biol 9(6):1418-1425. https://doi.org/10.1021/acssynbio.0c00096
Yu XH, Zhu XM, Lin XW, Li FW, Gu ZX (2016) Effects of two-stage controlled pH and temperature vs. one-step process for hemicellulase biosynthesis and feruloyl oligosaccharide fermentation using Aureobasidium pullulans. Bioresources 11(2):5113-5123.
Zambanini T, Kleineberg W, Sarikaya E, Buescher JM, Meurer G, Wierckx N, Blank LM (2016) Enhanced malic acid production from glycerol with high-cell density Ustilago trichophora TZ1 cultivations. Biotechnol Biofuels 9(135):1-10. https://doi.org/10.1186/s13068-016-0553-7
Zelle RM, de Hulster E, van Winden WA, de Waard P, Dijkema C, Winkler AA, Geertman JM, van Dijken JP, Pronk JT, van Maris AJ (2008) Malic acid production by Saccharomyces cerevisiae: engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl Environ Microbiol 74(9):2766-77. https://doi.org/10.1128/AEM.02591-07
Zhang X, Wang X, Shanmugam KT, Ingram LO (2011) L-malate production by metabolically engineered Escherichia coli. Appl Environ Microbiol 77(2):427–34. https://doi.org/10.1128/AEM.01971-10
Zhang ZY, Jin B, Kelly JM (2007) Production of lactic acid and byproducts from waste potato starch by Rhizopus arrhizus: role of nitrogen sources. World J Microbiol Biotechnol 23(2):229-236. https://doi.org/10.1007/s11274-006-9218-1
Zhao CG, Cheng LK, Xu QY, Wang J, Shen ZQ, Chen N (2015) Improvement of the production of L-tryptophan in Escherichia coli by application of a dissolved oxygen stage control strategy. Ann Microbiol 66(2):843–854. https://doi.org/10.1007/s13213-015-1172-4
Funding
This work was financially supported by the Key Program of the National Natural Science Foundation of China (22038005), the National Key R & D Program of China (2018YFA0901400, 2020YFA0908500), the General Program of National Natural Science Foundation of China (21978113), and the national first-class discipline program of Light Industry Technology and Engineering (LITE2018-08).
Author information
Authors and Affiliations
Contributions
Ji L and Luo Q conceived and designed research. Ji L and Wang J conducted experiments. Ji L analyzed the data and wrote the manuscript. Wang J, Ding Q, and Tang W contributed with scientific discussions and commented on the manuscript. Chen X and Liu L supervised the work and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies on human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ji, L., Wang, J., Luo, Q. et al. Enhancing L-malate production of Aspergillus oryzae by nitrogen regulation strategy. Appl Microbiol Biotechnol 105, 3101–3113 (2021). https://doi.org/10.1007/s00253-021-11149-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-021-11149-6