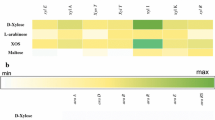
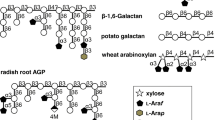
Abstract
Extracellular glycosyl hydrolases are uncommon in lactobacilli and include amylases and fructosidases mediating starch and fructan utilization, respectively. Extracellular arabinanases have not been described in lactobacilli. This study is aimed at identifying the function of an arabinan utilization operon in Lactobacillus crispatus DSM29598 and at characterizing two putative extracellular arabinanases that are located on that operon. The arabinan utilization operon of L. crispatus DSM29598 encodes enzymes for degradation of arabinan, α-galactosidases, β-galactosidases, and enzymes and for utilization of arabinose including phosphoketolase. The two putative extracellular arabinanases, AbnA and AbnB, are homologous to family GH43 endo-arabinanases. In Lactobacillaceae, homologs of these enzymes were identified exclusively in vertebrate-adapted species of the genus Lactobacillus. L. crispatus grew with arabinan from sugar beet pectin as sole carbon source, indicating extracellular arabinanase activity, and produced lactate and acetate, indicating metabolism via the phosphoketolase pathway. The two arabinanases AbnA and AbnB were heterologously expressed and purified by affinity chromatography. AbnA hydrolyzed linear and branched arabinan, while AbnB hydrolyzed only linear arabinan. The optimum pH for AbnA and AbnB was 6 and 7.5, respectively; 40 °C was the optimum temperature for both enzymes. The application of arabinan degrading L. crispatus as probiotic or as synbiotic with pectins may improve the production of short-chain fatty acids from pectin to benefit host health.
Key points
• An arabinan utilization operon in L. crispatus encodes two extracellular arabinanases.
• The same operon also encodes metabolic genes for arabinose conversion.
• In Lactobacillaceae, extracellular arabinanases are exclusive to Lactobacillus species.






Similar content being viewed by others
References
Antikainen J, Anton L, Sillanpää J, Korhonen TK (2002) Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Mol Microbiol 46:381–394. https://doi.org/10.1046/j.1365-2958.2002.03180.x
Åvall-Jääskeläinen S, Hynönen U, Ilk N, Pum D, Sleytr UB, Palva A (2008) Identification and characterization of domains responsible for self-assembly and cell wall binding of the surface layer protein of Lactobacillus brevis ATCC 8287. BMC Microbiol 8:165. https://doi.org/10.1186/1471-2180-8-165
Bindels LB, Delzenne NM, Cani PD, Walter J (2015) Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12:303–310. https://doi.org/10.1038/nrgastro.2015.47
Bouffard GG, Rudd KE, Adhya SL (1994) Dependence of lactose metabolism upon mutarotase encoded in the gal operon in Escherichia coli. J Mol Biol 244:269–278
De Angelis M, Bottacini F, Fosso B, Kelleher P, Calasso M, Di Cagno R, Ventura M, Picardi E, Van Sinderen D, Gobbetti M (2014) Lactobacillus rossiae, a vitamin B12 producer, represents a metabolically versatile species within the genus Lactobacillus. PLoS One 9:e207232. https://doi.org/10.1371/journal.pone.0107232
Deehan EC, Duar RM, Armet AM, Perez-Muñoz ME, Jin M, Walter J (2017) Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol Spectr 5:BAD-0019-2017. https://doi.org/10.1128/microbiolspec.bad-0019-2017
Dohm N, Petri A, Schlander M, Schlott B, König H, Claus H (2011) Molecular and biochemical properties of the S-layer protein from the wine bacterium Lactobacillus hilgardii B706. Arch Microbiol 193:251–261. https://doi.org/10.1007/s00203-010-0670-9
Duar RM, Lin XB, Zheng J, Martino ME, Grenier T, Pérez-Muñoz ME, Leulier F, Gänzle M, Walter J (2017) Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS Microbiol Rev 41:S27–S48. https://doi.org/10.1093/femsre/fux030
Duncan SH, Russell WR, Quartieri A, Rossi M, Parkhill J, Walker AW, Flint HJ (2016) Wheat bran promotes enrichment within the human colonic microbiota of butyrate-producing bacteria that release ferulic acid. Environ Microbiol 18:2214–2225. https://doi.org/10.1111/1462-2920.13158
Farro EGS, Leite AET, Silva IA, Filgueiras JG, de Azevedo ER, Polikarpov I, Nascimento AS (2018) GH43 endo-arabinanase from Bacillus licheniformis: structure, activity and unexpected synergistic effect on cellulose enzymatic hydrolysis. Int J Biol Macromol 117:7–16. https://doi.org/10.1016/j.ijbiomac.2018.05.157
Feldmann SD, Sahm H, Sprenger GA (1992) Cloning and expression of the genes for xylose isomerase and xylulokinase from Klebsiella pneumoniae 1033 in Escherichia coli K12. MGG Mol Gen Genet 234:201–210. https://doi.org/10.1007/BF00283840
Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA (2008) Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol 6:121–131. https://doi.org/10.1038/nrmicro1817
Fortina MG, Ricci G, Mora D, Guglielmetti S, Manachini PL (2003) Unusual organization for lactose and galactose gene clusters in Lactobacillus helveticus. Appl Environ Microbiol 69:3238–3243. https://doi.org/10.1128/AEM.69.6.3238-3243.2003
Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, Zhang M, Oh PL, Heng NCK, Patil PB, Juge N, MacKenzie DA, Pearson BM, Lapidus A, Dalin E, Tice H, Goltsman E, Land M, Hauser L, Ivanova N, Kyrpides NC, Walter J (2011) The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet 7:e1001314. https://doi.org/10.1371/journal.pgen.1001314
Fujisawa T, Benno Y, Yaeshima T, Mitsuoka T (1992) Taxonomic study of the Lactobacillus acidophilus group, with recognition of Lactobacillus gallinarum sp. nov. and Lactobacillus johnsonii sp. nov. and synonymy of Lactobacillus acidophilus group A3 (Johnson et al. 1980) with the type strain of Lactobacillus amylovorus (Nakamura 1981). Int J Syst Bacteriol 42:487–491. https://doi.org/10.1099/00207713-42-3-487
Fujita K, Takashi Y, Obuchi E, Kitahara K, Suganuma T (2014) Characterization of a novel β-l-arabinofuranosidase in Bifidobacterium longum: functional elucidation of a duf1680 protein family member. J Biol Chem 289:5240–5249. https://doi.org/10.1074/jbc.M113.528711
Gänzle MG (2015) Lactic metabolism revisited: metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr Opin Food Sci 2:106–117. https://doi.org/10.1016/j.cofs.2015.03.001
Gänzle MG, Follador R (2012) Metabolism of oligosaccharides and starch in lactobacilli: a review. Front Microbiol 3:e340. https://doi.org/10.3389/fmicb.2012.00340
Gänzle MG, Vogel RF (2003) Contribution of reutericyclin production to the stable persistence of Lactobacillus reuteri in an industrial sourdough fermentation. Int J Food Microbiol 80:31–45. https://doi.org/10.1016/S0168-1605(02)00146-0
Gigli-Bisceglia N, Engelsdorf T, Hamann T (2020) Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell Mol Life Sci 77:2049–2077
Hong MR, Park CS, Oh DK (2009) Characterization of a thermostable endo-1,5-α-L-arabinanase from Caldicellulorsiruptor saccharolyticus. Biotechnol Lett 31:1439–1443. https://doi.org/10.1007/s10529-009-0019-0
Ichinose H, Yoshida M, Fujimoto Z, Kaneko S (2008) Characterization of a modular enzyme of exo-1,5-α-L-arabinofuranosidase and arabinan binding module from Streptomyces avermitilis NBRC14893. Appl Microbiol Biotechnol 80:399–408. https://doi.org/10.1007/s00253-008-1551-x
Inácio JM, De Sá-Nogueira I (2008) Characterization of abn2 (yxiA), encoding a Bacillus subtilis GH43 arabinanase, Abn2, and its role in arabino-polysaccharide degradation. J Bacteriol 190:4272–4280. https://doi.org/10.1128/JB.00162-08
Inácio JM, Lopes Correia I, de Sá-Nogueira I (2008) Two distinct arabinofuranosidases contribute to arabino-oligosaccharide degradation in Bacillus subtilis. Microbiology 154:2719–2729. https://doi.org/10.1099/mic.0.2008/018978-0
Johnson B, Selle K, O’Flaherty S, Goh YJ, Klaenhammer T (2013) Identification of extracellular surface-layer associated proteins in Lactobacillus acidophilus NCFM. Microbiol (United Kingdom) 159:2269–2282. https://doi.org/10.1099/mic.0.070755-0
Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Bioinformatics 8:275–282. https://doi.org/10.1093/bioinformatics/8.3.275
Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F (2016) From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165:1332–1345. https://doi.org/10.1016/j.cell.2016.05.041
Koropatkin NM, Cameron EA, Martens EC (2012) How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10:323–335. https://doi.org/10.1038/nrmicro2746
Lebreton F, Willems RJL, Gilmore MS (2014) Enterococcus diversity, origins in nature, and gut colonization. In: Gilmore MS, Clewell DB, Ike Y, Shankar N (eds) Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston, pp 1–59
Lee YJ, Lee SJ, Kim SB, Lee SJ, Lee SH, Lee DW (2014) Structural insights into conserved L-arabinose metabolic enzymes reveal the substrate binding site of a thermophilic L-arabinose isomerase. FEBS Lett 588:1064–1070. https://doi.org/10.1016/j.febslet.2014.02.023
Leth ML, Ejby M, Workman C, Ewald DA, Pedersen SS, Sternberg C, Bahl MI, Licht TR, Aachmann FL, Westereng B, Hachem MA (2018) Differential bacterial capture and transport preferences facilitate co-growth on dietary xylan in the human gut. Nat Microbiol 3:570–580. https://doi.org/10.1038/s41564-018-0132-8
Li Q, Loponen J, Gänzle MG (2020) Characterization of the extracellular fructanase FruA in Lactobacillus crispatus and its contribution to fructan hydrolysis in breadmaking. J Agric Food Chem in press 68:8637–8647. https://doi.org/10.1021/acs.jafc.0c02313
Loponen J, Gänzle MG (2018) Use of sourdough in low FODMAP baking. Foods 7:96. https://doi.org/10.3390/foods7070096
Loponen J, Mikola M, Sibakov J (2017) An enzyme exhibiting fructan hydrolase activity. WO 2017/220864 A1
Meng J, Jin D, Yang J, Lai X-H, Pu J, Zhu W, Huang Y, Liang H, Lu S (2020) Lactobacillus xujianguonis sp. nov., isolated from faeces of Marmota himalayana. Int J Syst Evol Microbiol 70:11–15. https://doi.org/10.1099/ijsem.0.003598
Nguyen TH, Splechtna B, Krasteva S, Kneifel W, Kulbe KD, Divne C, Haltrich D (2007) Characterization and molecular cloning of a heterodimeric β-galactosidase from the probiotic strain Lactobacillus acidophilus R22. FEMS Microbiol Lett 269:136–144. https://doi.org/10.1111/j.1574-6968.2006.00614.x
Orla-Jensen S (1919) The lactic acid bacteria. Andr Fred Høst and Son, Copenhagen
Overbeeke N, Fellinger AJ, Toonen MY, van Wassenaar D, Verrips CT (1989) Cloning and nucleotide sequence of the α-galactosidase cDNA from Cyamopsis tetragonoloba (guar). Plant Mol Biol 13:541–550. https://doi.org/10.1007/BF00027314
Pontonio E, Mahony J, Di Cagno R, O’Connell Motherway M, Lugli GA, O’Callaghan A, De Angelis M, Ventura M, Gobbetti M, van Sinderen D (2016) Cloning, expression and characterization of a β-d-xylosidase from Lactobacillus rossiae DSM 15814T. Microb Cell Factories 15:1–13. https://doi.org/10.1186/s12934-016-0473-z
Posthuma CC, Bader R, Engelmann R, Postma PW, Hengstenberg W, Pouwels PH (2002) Expression of the xylulose 5-phosphate phosphoketolase gene, xpkA, from Lactobacillus pentosus MD363 is induced by sugars that are fermented via the phosphoketolase pathway and is repressed by glucose mediated by CcpA and the mannose phosphoenolpyrvate phosphotransferase system. Appl Environ Microbiol 68:831–837. https://doi.org/10.1128/AEM.68.2.831
Pot B, Felis G, De Bruyne K, Tsakalidou E, Papadimitriou K, Leisner J, Vandamme P (2014) The genus Lactobacillus. In: Holzapfel W, Wood B (eds) Lactic acid bacteria: biodiversity and taxonomy. John Wiley & Sons, Inc, Hoboken, pp 249–353
Rhimi M, Ilhammami R, Bajic G, Boudebbouze S, Maguin E, Haser R, Aghajari N (2010) The acid tolerant L-arabinose isomerase from the food grade Lactobacillus sakei 23K is an attractive D-tagatose producer. Bioresour Technol 101:9171–9177. https://doi.org/10.1016/j.biortech.2010.07.036
Ridley BL, O’Neill MA, Mohnen D (2001) Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry 57:929–967. https://doi.org/10.1016/S0031-9422(01)00113-3
Rumpagaporn P, Reuhs BL, Kaur A, Patterson JA, Keshavarzian A, Hamaker BR (2015) Structural features of soluble cereal arabinoxylan fibers associated with a slow rate of in vitro fermentation by human fecal microbiota. Carbohydr Polym 130:191–197. https://doi.org/10.1016/j.carbpol.2015.04.041
Russell RRB, Opoku JA, Sutcliffe IC, Tao L, Ferretti JJ (1992) A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J Biol Chem 267:4631–4637
Sá-Nogueira I, Nogueira TV, Soares S, De Lencastre H (1997) The Bacillus subtilis L-arabinose (ara) operon: nucleotide sequence, genetic organization and expression. Microbiology 143:957–969. https://doi.org/10.1099/00221287-143-3-957
Saulnier L, Sado PE, Branlard G, Charmet G, Guillon F (2007) Wheat arabinoxylans: exploiting variation in amount and composition to develop enhanced varieties. J Cereal Sci 46:261–281. https://doi.org/10.1016/j.jcs.2007.06.014
Shallom D, Belakhov V, Solomon D, Gilead-Gropper S, Baasov T, Shoham G, Shoham Y (2002) The identification of the acid-base catalyst of α-arabinofuranosidase from Geobacillus stearothermophilus T-6, a family 51 glycoside hydrolase. FEBS Lett 514:163–167. https://doi.org/10.1016/S0014-5793(02)02343-8
Shi H, Ding H, Huang Y, Wang L, Zhang Y, Li X, Wang F (2014) Expression and characterization of a GH43 endo-arabinanase from Thermotoga thermarum. BMC Biotechnol 14:1–9. https://doi.org/10.1186/1472-6750-14-35
Shulami S, Raz-Pasteur A, Tabachnikov O, Gilead-Gropper S, Shner I, Shoham Y (2011) The L-arabinan utilization system of Geobacillus stearothermophilus. J Bacteriol 193:2838–2850. https://doi.org/10.1128/JB.00222-11
Sillanpää J, Martinez B, Antikainen J, Toba T, Kalkkinen N, Tankka S, Lounatmaa K, Keränen J, Höök M, Westerlund-Wikström B, Pouwels PH, Korhonen TK (2000) Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J Bacteriol 182:6440–6450
Smit E, Oling F, Demel R, Martinez B, Pouwels PH (2001) The S-layer protein of Lactobacillus acidophilus ATCC 4356: identification and characterisation of domains responsible for S-protein assembly and cell wall binding. J Mol Biol 305:245–257. https://doi.org/10.1006/jmbi.2000.4258
Squina FM, Santos CR, Ribeiro DA, Cota J, de Oliveira RR, Ruller R, Mort A, Murakami MT, Prade RA (2010) Substrate cleavage pattern, biophysical characterization and low-resolution structure of a novel hyperthermostable arabinanase from Thermotoga petrophila. Biochem Biophys Res Commun 399:505–511. https://doi.org/10.1016/j.bbrc.2010.07.097
Stecher G, Tamura K, Kumar S (2020) Molecular evolutionary genetics analysis (MEGA) for macOS. Mol Biol Evol 37:1237–1239. https://doi.org/10.1093/molbev/msz312/5697095
Tang KX, Zhao CJ, Gänzle MG (2017) Effect of glutathione on the taste and texture of type I sourdough bread. J Agric Food Chem 65:4321–4328. https://doi.org/10.1021/acs.jafc.7b00897
van Hijum SAFT, Kralj S, Ozimek LK, Dijkhuizen L, van Geel-Schutten IGH (2006) Structure-function relationships of glucansucrase and fructansucrase enzymes from lactic acid bacteria. Microbiol Mol Biol Rev 70:157–176. https://doi.org/10.1128/mmbr.70.1.157-176.2006
von Heijne G (1990) The signal peptide. J Membr Biol 115:195–201
Walter J (2008) Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol 74:4985–4996. https://doi.org/10.1128/AEM.00753-08
Willats WGT, Mccartney L, Mackie W, Knox JP (2001) Pectin: cell biology and prospects for functional analysis. Plant Mol Biol 47:9–27. https://doi.org/10.1023/A:1010662911148
Yan YL, Hu Y, Gänzle MG (2018) Prebiotics, FODMAPs and dietary fiber — conflicting concepts in development of functional food products? Curr Opin Food Sci 20:30–37. https://doi.org/10.1016/j.cofs.2018.02.009
Yong JG, Lee JH, Hutkins RW (2007) Functional analysis of the fructooligosaccharide utilization operon in Lactobacillus paracasei 1195. Appl Environ Microbiol 73:5716–5724. https://doi.org/10.1128/AEM.00805-07
Yoshida K, Shindo K, Sano H, Seki S, Fujimura M, Yanai N, Miwa Y, Fujita Y (1996) Sequencing of a 65 kb region of the Bacillus subtilis genome containing the lic and cel loci, and creation of a 177 kb contig covering the gnt-sacXY region. Microbiology 142:3113–3123
Zhao C, Pyle AM (2017) The group II intron maturase: a reverse transcriptase and splicing factor go hand in hand. Curr Opin Struct Biol 47:30–39. https://doi.org/10.1016/j.gde.2016.03.011
Zhao X, Gänzle MG (2018) Genetic and phenotypic analysis of carbohydrate metabolism and transport in Lactobacillus reuteri. Int J Food Microbiol 272:12–21. https://doi.org/10.1016/j.ijfoodmicro.2018.02.021
Zheng J, Ruan L, Sun M, Gänzle MG (2015) A genomic view of lactobacilli and pediococci demonstrates that phylogeny matches ecology and physiology. Appl Environ Microbiol 81:7233–7243. https://doi.org/10.1128/AEM.02116-15
Zheng J, Wittouck S, Salvetti E, Franz CMAB, Harris HMB, Mattarelli P, O’Toole PW, Pot B, Vandamme P, Walter J, Watanabe K, Wuyts S, Felis GE, Gänzle MG, Lebeer S (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858. https://doi.org/10.1099/ijsem.0.004107
Acknowledgments
Reviewer #2 is acknowledged for critical comments that improved the interpretation of experimental results.
Funding
Funding was provided by the Alberta Wheat Commission, the Saskatchewan Wheat Development Commission, the Minnesota Wheat Research and Promotion Council (grant no. 2018F031R), and the Natural Sciences and Engineering Research Council of Canada (NSERC, grant no. CRDPJ542616-19). Q.L. acknowledges stipend support from the China Scholarship Council, and M.G. acknowledges the Canada Research Chairs program.
Author information
Authors and Affiliations
Contributions
QL and MGG conceived and designed research. QL conducted experiments; QL and MGG wrote the manuscript and read and approved the final manuscript version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 607 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Gänzle, M.G. Characterization of two extracellular arabinanases in Lactobacillus crispatus. Appl Microbiol Biotechnol 104, 10091–10103 (2020). https://doi.org/10.1007/s00253-020-10979-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10979-0