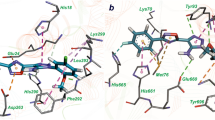
Abstract
Increasing drug resistance in pathogens including Mycobacterium tuberculosis (MTB) has been ascribed to mutations in the known target genes. However, many of these drugs have multiple targets; some of which have not been identified so far. Understanding the mechanism of action of these drugs holds a great promise in better management of disease especially by drug-resistant strains. In this study, we report glutamate racemase (MurI), a crucial enzyme of phase I peptidoglycan (PG) biosynthesis pathway of MTB, as an additional target of ethambutol (EMB). The effect on EMB on the MurI protein at structural and functional level was studied using different spectroscopic, biochemical, and insilico approaches. Spectroscopic analysis revealed that EMB-modified protein undergoes conformational alterations. Furthermore, in vitro racemization studies of the MurI protein suggest that EMB decreases its functional activity. Docking studies revealed that EMB interacts with most of the active residues at the binding site and blocks the binding pocket. Overall, data suggests that EMB, a primary drug used for the treatment of tuberculosis (TB), acts as a competitive inhibitor of substrate for binding to mycobacterial MurI protein. The study also points out to our lacunae in understanding the site and mechanism of action of existing drugs. Furthermore, glutamate racemase is a conserved protein of the bacterial kingdom; therefore, ethambutol could be a promising candidate as a broad-spectrum antibiotic for many other bacterial diseases.




Similar content being viewed by others
References
Alvarez HA, McCarthy AN, Grigera JR (2012) A molecular dynamics approach to ligand-receptor interaction in the aspirin-human serum albumin complex. J Biophys 2012:1–7. https://doi.org/10.1155/2012/642745
Amin AG, Goude R, Shi L, Zhang J, Chatterjee D, Parish T (2008) EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis. Microbiology 154:240–248. https://doi.org/10.1099/mic.0.2007/012153-0
Belanger AE, Besra GS, Ford ME, Mikusova K, Belisle JT, Brennan PJ, Inamine JM (1996) The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc Natl Acad Sci 93:11919–11924. https://doi.org/10.1073/pnas.93.21.11919
Bugg TD, Walsh CT (1992) Intracellular steps of bacterial cell wall peptidoglycan biosynthesis: enzymology, antibiotics, and antibiotic resistance. Nat Prod Rep 9:199–215
Castonguay F, Dufour JJ, Minvielle F, Estrada R (1990) Follicular dynamics and dominance in Booroola x Finnish Landrace and Booroola x Suffolk ewes heterozygous for the F gene. J Reprod Fertil 89:193–203
Chen YH, Yang JT, Chau KH (1974) Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry (Mosc) 13:3350–3359
Epand RM, Walker C, Epand RF, Magarvey NA (2016) Molecular mechanisms of membrane targeting antibiotics. Biochim Biophys Acta BBA - Biomembr 1858:980–987. https://doi.org/10.1016/j.bbamem.2015.10.018
Fisher SL (2008) Glutamate racemase as a target for drug discovery. Microb Biotechnol 1:345–360. https://doi.org/10.1111/j.1751-7915.2008.00031.x
Gagnon JK, Law SM, Brooks III CL (2014) Flexible CDOCKER: development and application of a pseudo-explicit structure-based docking method within CHARMM. Biophys J 106:646a . doi: https://doi.org/10.1016/j.bpj.2013.11.3576
Gallo KA, Knowles JR (1993) Purification, cloning, and cofactor independence of glutamate racemase from Lactobacillus. Biochemistry (Mosc) 32:3981–3990
Gallo KA, Tanner ME, Knowles JR (1993) Mechanism of the reaction catalyzed by glutamate racemase. Biochemistry (Mosc) 32:3991–3997. https://doi.org/10.1021/bi00066a020
Glavas S, Tanner ME (1999) Catalytic acid/base residues of glutamate racemase †. Biochemistry (Mosc) 38:4106–4113. https://doi.org/10.1021/bi982663n
Glavas S, Tanner ME (2001) Active site residues of glutamate racemase †. Biochemistry (Mosc) 40:6199–6204. https://doi.org/10.1021/bi002703z
Goude R, Amin AG, Chatterjee D, Parish T (2009) The arabinosyltransferase EmbC is inhibited by ethambutol in Mycobacterium tuberculosis. Antimicrob Agents Chemother 53:4138–4146. https://doi.org/10.1128/AAC.00162-09
Hasan T, Ali M, Saluja D, Singh LR (2015) pH might play a role in regulating the function of paired amphipathic helices domains of human Sin3B by altering structure and thermodynamic stability. Biochem Biokhimiia 80:424–432. https://doi.org/10.1134/S0006297915040057
Hay M, Thomas DW, Craighead JL, Economides C, Rosenthal J (2014) Clinical development success rates for investigational drugs. Nat Biotechnol 32:40–51. https://doi.org/10.1038/nbt.2786
Hess B (2008) P-LINCS: a parallel linear constraint solver for molecular simulation. J Chem Theory Comput 4:116–122. https://doi.org/10.1021/ct700200b
Hwang KY, Cho CS, Kim SS, Sung HC, Yu YG, Cho Y (1999) Structure and mechanism of glutamate racemase from Aquifex pyrophilus. Nat Struct Biol 6:422–426. https://doi.org/10.1038/8223
Lee JY (2015) Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc Respir Dis 78:47–55. https://doi.org/10.4046/trd.2015.78.2.47
Liu Y, Breukink E (2016) The membrane steps of bacterial cell wall synthesis as antibiotic targets. Antibiotics 5:28. https://doi.org/10.3390/antibiotics5030028
Lundqvist T, Fisher SL, Kern G, Folmer RHA, Xue Y, Newton DT, Keating TA, Alm RA, de Jonge BLM (2007) Exploitation of structural and regulatory diversity in glutamate racemases. Nature 447:817–822. https://doi.org/10.1038/nature05689
Petersen HG (1995) Accuracy and efficiency of the particle mesh Ewald method. J Chem Phys 103:3668–3679. https://doi.org/10.1063/1.470043
Poen S, Nakatani Y, Opel-Reading HK, Lasse M, Dobson RCJ, Krause KL (2016) Exploring the structure of glutamate racemase from Mycobacterium tuberculosis as a template for anti-mycobacterial drug discovery. Biochem J 473:1267–1280. https://doi.org/10.1042/BCJ20160186
Pronk S, Páll S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29:845–854. https://doi.org/10.1093/bioinformatics/btt055
Raviglione M, Sulis G (2016) Tuberculosis 2015: burden, challenges and strategy for control and elimination. Infect Dis Rep 8. https://doi.org/10.4081/idr.2016.6570
Ruzheinikov SN, Taal MA, Sedelnikova SE, Baker PJ, Rice DW (2005) Substrate-induced conformational changes in Bacillus subtilis glutamate racemase and their implications for drug discovery. Struct Lond Engl 1993 13:1707–1713. https://doi.org/10.1016/j.str.2005.07.024
Sengupta S, Ghosh S, Nagaraja V (2008) Moonlighting function of glutamate racemase from Mycobacterium tuberculosis: racemization and DNA gyrase inhibition are two independent activities of the enzyme. Microbiology 154:2796–2803. https://doi.org/10.1099/mic.0.2008/020933-0
Shehzad A, Rehman G, Ul-Islam M, Khattak WA, Lee YS (2013) Challenges in the development of drugs for the treatment of tuberculosis. Braz J Infect Dis 17:74–81. https://doi.org/10.1016/j.bjid.2012.10.009
Silhavy TJ, Kahne D, Walker S (2010) The bacterial cell envelope. Cold Spring Harb Perspect Biol 2:a000414. https://doi.org/10.1101/cshperspect.a000414
Smith I (2003) Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin Microbiol Rev 16:463–496. https://doi.org/10.1128/CMR.16.3.463-496.2003
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Sulis G, Centis R, Sotgiu G, D’Ambrosio L, Pontali E, Spanevello A, Matteelli A, Zumla A, Migliori GB (2016) Recent developments in the diagnosis and management of tuberculosis. Npj Prim Care Respir Med 26:16078. https://doi.org/10.1038/npjpcrm.2016.78
Szabo AG, Lynn KR, Krajcarski DT, Rayner DM (1978) Tyrosinate fluorescence maxima at 345 nm in proteins lacking tryptophan at pH 7. FEBS Lett 94:249–252
Tanner ME, Gallo KA, Knowles JR (1993) Isotope effects and the identification of catalytic residues in the reaction catalyzed by glutamate racemase. Biochemistry (Mosc) 32:3998–4006
Tanwar O, Deora GS, Tanwar L, Kumar G, Janardhan S, Alam M, null S, Akhter M (2014) Novel hydrazine derivatives as selective DPP-IV inhibitors: findings from virtual screening and validation through molecular dynamics simulations. J Mol Model 20:2118. https://doi.org/10.1007/s00894-014-2118-7
Telenti A, Philipp WJ, Sreevatsan S, Bernasconi C, Stockbauer KE, Wieles B, Musser JM, Jacobs WR (1997) The emb operon, a gene cluster of Mycobacterium tuberculosis involved in resistance to ethambutol. Nat Med 3:567–570
Teo A, Roper D (2015) Core steps of membrane-bound peptidoglycan biosynthesis: recent advances, insight and opportunities. Antibiotics 4:495–520. https://doi.org/10.3390/antibiotics4040495
van Aalten DM, Bywater R, Findlay JB, Hendlich M, Hooft RW, Vriend G (1996) PRODRG, a program for generating molecular topologies and unique molecular descriptors from coordinates of small molecules. J Comput Aided Mol Des 10:255–262
Walsh CT (1989) Enzymes in the D-alanine branch of bacterial cell wall peptidoglycan assembly. J Biol Chem 264:2393–2396
Acknowledgements
The authors thank the Department of Science and Technology (DST) purse grant and MG grant to DS for financial support. The support from Department of Biotechnology, Govt. of India for Bioinformatics Facility at Dr. B.R. Ambedkar Center for Biomedical Research is highly acknowledged. Senior research fellowship to AP from Indian Council for Medical Research and DST-INSPIRE fellowship to PJ from DST is gratefully acknowledged.
Funding
The project grants, purse grant and MG grant, were received by DST and University of Delhi. Fellowship and contingency grant was provided to AP from ICMR and PJ from DST.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 766 kb)
Rights and permissions
About this article
Cite this article
Pawar, A., Jha, P., Konwar, C. et al. Ethambutol targets the glutamate racemase of Mycobacterium tuberculosis—an enzyme involved in peptidoglycan biosynthesis. Appl Microbiol Biotechnol 103, 843–851 (2019). https://doi.org/10.1007/s00253-018-9518-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9518-z