Abstract
Brucella as intracellular pathogen requires a coordinate interaction between Th1 subset of gamma interferon-secreting CD4 T cells and CD8 T cells for optimal protective immunity. It was previously recognized that L7/L12 as T cell-reactive antigen from the pathogen. On other hand, Omp25 was found as another antigen to provide protection against the Brucella infection by eliciting both Th1 and Th2 type of immune responses in mice. Here, we analyzed the prophylactic and therapeutic efficacy of a divalent fusion protein (rL7/L12-Omp25) comprising these two promising immunogens of Brucella in the presence of murine IFN-gamma in mice against B. abortus 544 challenge. rIFN-gamma with rL7/L12-Omp25 resulted in superior immune response when compared to the animal vaccine strain B. abortus S19. The vaccine candidate caused dominance of IgG1 over IgG2a and upregulated cytokine secretion (IFN-gamma, TNF-α, and IL-10) among immunized mice. Moreover, the antigen in combination with murine IFN-gamma elicited stronger cell-mediated immune response among the immunized animals when compared to standard vaccine (S19). The registered log protection unit among challenged mice with B. abortus 544 pathogen was 2.16, p = 0.0001 when rL7/L12-Omp25 was administered alone and 2.4, p = 0.0001 when it was administered along with rIFN-gamma. However, the molecule upon administration with murine IFN-gamma imparted very minimal or no therapeutic effect against brucellosis. To conclude, our study demonstrates the potential of rL7/L12-Omp25 as an immunogen of prospective and efficient prophylaxis as it is capable of eliciting both cell-mediated and humoral immune responses against brucellosis.
Similar content being viewed by others
Introduction
Protective immunity among susceptible hosts against intracellular facultative bacterial pathogens such as Brucella abortus is mediated by cellular and humoral immune response. Activation of CD4+ and CD8+ T lymphocyte subpopulation is required for clearance of this pathogen (Araya et al. 1989; Schurig et al. 2002). Besides, induction of interferon-gamma has a crucial role in upregulating macrophage cells for enhanced production of reactive oxygen intermediates to control the infection (Stevens et al. 1992).
Live attenuated strains such as Brucella abortus S19 are known to induce strong cell-mediated immune responses and hence being used as vaccine strategies in many countries. This attenuated smooth strain of Brucella is very effective in conferring protection against virulent strains of B. abortus. However, the attenuated strain is still far from the ideal vaccine strategy as it causes abortion in pregnant animals also known to be pathogenic for humans (Moriyón et al. 2004). As an alternative, scientists have turned towards recombinant subunit antigens of Brucella to develop promising and safe vaccine molecules (O’Garra and Murphy 1994; Oliveira et al. 1994; Tabatabai and Pugh 1994; Toth et al. 1995; Lin et al. 1996; Denoel et al. 1997; De Fays et al. 1999; Baloglu et al. 2000; Goel and Bhatnagar 2012). Several subunit candidate vaccine molecules are being developed and studied to confer moderate to satisfactory protection against brucellosis in clinical trials.
Earlier studies have already proven that L7/L12 is a strong immunogen from this pathogen and induces CD4+ T cell response with significant level of IFN-gamma production in murine model (Oliveira and Splitter 1994). Although, L7/L12 is recognized as a T cell-reactive antigen from the pathogen, it was found to induce moderate protection against B. abortus infection in mouse model (Oliveira and Splitter 1996; Kurar and Splitter 1997). This entails that vaccine potential of monovalent rL7/L12 can be improved with combination of other proteins that conferred resistance against the pathogen with efficient protective immune response than immunization with the individual components. Another protein Omp25 is an immunogen of Brucella proven to possess more effective vaccine potential in murine model by eliciting both Th1 and Th2 type of immune responses (Goel and Bhatnagar 2012).
Certain factors towards vaccine development act as driving forces for effective vaccine formulation such as intrinsic immunological properties of an antigen, induced cytokines, route of administration, and dosage with suitable adjuvant properties. We have also noticed that supplementation of rIFN-gamma enhanced the host resistance against the pathogen and reduced bacterial load about tenfold in number of bacteria post 1-week infection (Stevens et al. 1992; Zhan and Cheers 1993; Fernandes and Baldwin 1995).
Previous studies also have shown that combination or chimera of some of the Brucella antigens induces protective immune response better than with the individual components (Luo et al. 2006; Cassataro et al. 2007; Tadepalli et al. 2016). These observations further encouraged us to study efficacy of a chimeric divalent protein comprising L7/L12 and Omp25 in the presence of murine gamma interferon against B. abortus 544 infection in mice model. We adopted intraperitoneal route of immunization as it gives a larger absorptive surface area for a vaccine administered. Furthermore, mice infected with virulent strain of B. abortus 544 were used to evaluate the therapeutic efficacy of the chimeric molecule in the presence of rIFN-gamma.
Materials and methods
Bacterial propagation
Live attenuated vaccine strain S19 and virulent strain 544 (ATCC 23448) of Brucella abortus were obtained from the repository of the Indian Veterinary Research Institute, Izatnagar, Bareilly, India. The strains were grown in Tryptic soy medium using Brucella antibiotics (Sigma, India) as per manufacturer’s instructions. Strains of E. coli namely DH5α and BL21(DE3) pLysS (Novagen, Madison, WI) were used for cloning and expression studies and were grown in Luria Bertani (LB) medium (Himedia, India). Antibiotics used for bacterial propagation include 100 μg/ml for ampicillin and 35 μg/ml for chloramphenicol.
Mice
Five to 6 weeks of inbred female BALB/c mice confirmed as specific pathogen-free were collected from the Central Animal Facility of Defence Food Research Laboratory (DFRL) and housed in standard cages (n = 10 mice/cage) under pathogen-free condition and provided water and fed ad libitum. Mice were acclimatized to housing for a week under repeated monitoring before initiating the experiments. The protection experiments were performed in biosafety level 3 animal facilities. The animal experiments were carried out with permit no SS-10/DRD (DOM)-001.22/DFR (ST) by following the standards of Institutional Animal Ethics committee (IAEC) and institutional regulations.
Gene target identification
Whole genome sequence of B. abortus S19 strain (accession no. CP000887) available in the National Center for Biotechnology Information (NCBI) database was used for the selection of genes encoding immunodominant virulence factors. The 50S ribosomal protein-encoding gene of L7/L12 (accession no. BAbS19_I11800) and Omp25 (accession no. BAbS19_I06750) were selected as potential targets for development of a candidate divalent vaccine molecule.
L7/L12-Omp25 fusion protein preparation
Nucleotide sequences of L7/L12 and Omp25 encoding genes were retrieved from GenBank for designing cloning primers. Coding regions of both antigens excluding signal peptide were spliced via glycine linker (G4S) by restriction ligation method using the primers are as follows: L7/L12F: CGCGGATCCATGGCTGATCTCGCAAAGAT, L7/L12R: TCCCTGCAGAGAACCACCACCCTTGAGTTCAACCTTGGCG, Omp25F: GGACTGCAGGCCGACGCCATCC and Omp25R: CCGAAGCTTTTAGAACTTGTAGCCGATGCC. Sequences underlined are restriction sites and bold letters represent G4S stretch incorporated in the target gene amplicons during PCR. Primers used for PCR amplification of L7/L12 were designed to incorporate BamHI and G4S with PstI restriction sites at 5′ and 3′ end respectively. Similarly, primers used for PCR amplification of Omp25 included PstI and HindIII restriction sites at 5′ and 3′ end respectively. The PstI restriction site was incorporated to allow in-frame chimerization of L7/L12 and Omp25 PCR amplicons followed by restriction and ligation resulting in the chimeric fragment L7/L12-G4S-Omp25. PCR condition for amplification of both L7/L12 and Omp25 included an initial denaturation of 3 min at 95 °C followed by 30 cycles of denaturation at 95 °C for 1 min, annealing at 56 °C for 1 min and extension at 72 °C for 1 min, and a final extension of 8 min at 72 °C. The chimeric fragment obtained by restriction and ligation of both the gene targets was inserted into pRSET A vector (Invitrogen, USA) and resultant recombinant plasmid was confirmed for correct in-frame fusion of the target genes by DNA sequencing. The confirmed recombinant plasmid was transformed to E. coli BL21(DE3)pLysS. High-level expression of the rL7/L12-Omp25 fusion protein was achieved using of isopropyl-D-thiogalactoside (IPTG) and then purified by affinity column chromatography using Ni2+-NTA column (QIAGEN Inc.) and 8 M urea as described elsewhere (Estein et al. 2003). Eluted protein was confirmed by denatured SDS-PAGE and Western blotting. Further, the protein pool was refolded using 0.4 M L-arginine containing refolding buffer by gradient dialysis at 4 °C (Thomson et al. 2012).
Immunization of mice
Acclimatized BALB/c mice were made into four groups (n = 10), anesthetized with sodium pentobarbital anesthesia, and immunization was performed. Briefly, first group of mice received 30 μg of the fusion protein emulsified in alum (Sigma, India) intraperitoneally followed by a booster dose on the 15th day with the same amount of protein emulsified in alum. Simultaneously, group 2 received the same dosage of rL7/L12-Omp25 in cocktail with 10 μg of murine recombinant IFN-γ emulsified in alum in booster dose (Biolegend, San Diego, CA). Group 3 served as positive control and was intraperitoneally immunized with 5 × 105 CFU of live B. abortus vaccine strain S19 once at day 0 and the fourth group injected with sterile 1× PBS (phosphate-buffered saline, pH 7.4) served as negative control. Blood was collected by retro-orbital bleeding on 0, 15, 30, and 75 days of immunization schedule. Serum was separated from blood by centrifuging at 1650xg for 15 min and stored them at − 80 °C until use. The immunization experiment was repeated twice independently.
Evaluation of hyper immune sera for specific antibodies and their isotypes by indirect ELISA
In order to determine the antigen-specific antibody titer, an indirect enzyme-linked immunosorbent assay was performed as described (Goel and Bhatnagar 2012) using rL7/L12-Omp25, B. abortus S19, and PBS-immunized mice sera collected during intervals of days 0, 15, 30, and 75. Briefly, 96-well micro-titer plates (Catalog no. 475094, Thermo Scientific) were coated overnight at 4 °C with purified rL7/L12-Omp25 in 50 mM carbonate/bicarbonate buffer (pH 9.6) at a final concentration of 1 μg/well. Further, plates were blocked with 5% skimmed milk (Sigma, India) in 1× PBS (w/v). Later, the plates were incubated with 100 μl/well of twofold serial dilutions of serum in 1× PBS from 1:1000 to 1:256000 for 1 h at 37 °C. Plates were washed with 1× PBS-T (0.05% of Tween 20) for four times after each step. Horse radish peroxidase (HRP) conjugated goat-anti-mouse-IgG (Sigma, India) in addition with 200 μmol of o-phenylenediamine and 0.04% H2O2 were used as development of the assay. Sera from five individual mice from each immunization group were analyzed in triplicates. In addition, immunoglobulin isotyping for mice sera was determined from each group using ELISA based isotyping kit (ISO-2KT, Sigma, USA). Titers were determined by employing end-point dilution method as the highest dilution of serum having a mean OD two times greater than naive serum samples.
Lymphocyte proliferation assay
For determining the lymphocyte proliferation response, single-splenocyte suspensions in triplicates on the 30th post-immunization from each immunization group were prepared from the spleens of mice from each group. Briefly, splenocyte suspensions were subjected to erythrocyte lysis using ACK lysis buffer (Catalog no. A1049201, Thermo Fisher, India) and washed thrice with 1× PBS containing penicillin (100 U/ml) and gentamicin (30 μg/ml). Thus, obtained splenocytes were re-suspended in Dulbecco’s Modified Eagle Medium (Catalog no. D5546, Sigma, India) supplemented with 10% fetal bovine serum (Sigma, India) and seeded in a 96-well flat-bottom plate (4 × 105 viable cells/well). For stimulation, 30 μg/ml of rL7/L12-Omp25 was also added to each well and the splenocytes were cultured for 72 h. Concanavalin A was used as positive control at a concentration of 3 μg/ml. Splenocytes without any antigen treatment for stimulation served as negative control. After incubation period, proliferation response of the cells against exposed antigens was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) method as described elsewhere (Goel and Bhatnagar 2012). Supernatants collected during the assay were subjected to total protein concentrations and preserved at − 20 °C for cytokine production study. The proliferation was expressed as stimulation index (SI) calculated as
Cytokine analysis
The profile of cytokines induced by rL7/L12-Omp25 was determined using the culture supernatant of re-stimulated splenocytes of rL7/L12-Omp25, rL7/L12-Omp25 + rIFN-γ, B. abortus S19, and sham-immunized mice collected from the above experiment. Bio-Plex Pro Mouse Cytokine 8-plex assay kit (Catalog no. M60000007A, Bio-Rad, California) was used for quantification of cytokine level in the induced culture supernatant. The cytokine targets of detection were IFN-γ, TNF-α, IL-4, IL-5, IL-10, IL-2, IL-12(p70), and GM-CSF. The assay was performed in multiplex format along with appropriate standards as per the instructions provided in the assay kit using Bio-Plex HTF (Bio-Rad, California). Analysis of the obtained data was performed using Bio-Plex Manager, version 5.0 (Bio-Rad, California). Sample showing out of range (OOR) concentration was considered as the highest extrapolated value for a conservative estimate and statistical analysis. Standard curve was generated for standards to use as reference for determination of concentration of each cytokine in analyzed samples. The experiment was performed thrice with induced culture supernatant from rL7/L12-Omp25, rL7/L12-Omp25 + rIFN-γ, B. abortus S19, and sham-immunized mice.
Flow cytometry
To determine the B cell and T cell subpopulation difference among rL7/L12-Omp25 and sham-immunized mice, expression of CD3, CD4, CD8, and CD45/B220 surface markers on lymphocytes from all four groups was assessed by cell surface staining followed by flow cytometry. Samples were prepared using blood from each mice group collected 30 days after completion of immunization. Briefly, heparinized whole blood was incubated with appropriate fluorochrome-conjugated antibodies specific to the target surface markers. FITC anti-mouse CD45/B220 antibody (Catalog no. 103205), PE anti-mouse CD3 antibody (Catalog no. 100205), FITC anti-mouse CD4 antibody (Catalog no. 100405), and PE anti-mouse CD8a antibody (Catalog no. 100707) were used for staining the surface markers as per manufacturer’s recommended concentrations (Biolegend, San Diego, CA). After staining for 15 min at room temperature, the RBCs were lysed using FACS lysis solution and lymphocytes were centrifuged and washed with PBS for analysis. The cell count acquisition for the frequency of CD3+ and CD45/B220+ and CD4+ and CD8+ was performed using two-color fluorescence flow cytometric analysis of BD FACS Calibur system. The acquisition data (approximately 500,000 lymphocytes per sample) were analyzed using FlowJo v.10.1 software (Treestar, Inc., San Carlos, CA). This experiment was repeated in biological triplicates of the blood samples collected from all four mice groups on the 30th day of completion of immunization.
Protection experiments
Two weeks post-immunization, mice from each group were challenged intraperitoneally with virulent B. abortus 544 as described previously (Al-Mariri et al. 2001). Briefly, mice were challenged with 5 × 107 CFU of B. abortus 544 in 100 μl of PBS. After 30 days of infection, the infected mice were euthanized; spleen and livers were aseptically removed and homogenized in 2 ml of PBST (Sigma, India). The homogenate of both the organs was serially diluted and aliquots were plated on selective media and incubated at 37 °C with 10% CO2 for 48–72 h to determine the count of B. abortus 544 in spleen and liver of mice from all four groups. B. abortus 544 was differentiated from B. abortus S19 strains by subtracting the Brucella CFUs obtained under selective culturing in TSA containing 0.1% erythritol from CFUs obtained under non-selective culturing in TSA only (Delpino et al. 2007). Thus, derived CFU values were converted to logarithmic values. Unit of protection conferred by rL7/L12-Omp25 in presence and absence of rIFN-γ was obtained by subtracting the mean log10CFU of the experimental group from that of the corresponding negative control group (Cassataro et al. 2005).
Therapeutic treatment of B. abortus 544-infected mice
Five to 6-week-old female mice were obtained and divided into three groups. Six female mice in each group were infected with 5 × 107 CFU of live B. abortus 544 intraperitoneally. On the 30th day after infection, the group one mice were treated with 30 μg of rL7/L12-Omp25 and 10 μg of rIFN-γ. On the other hand, the group two mice were treated with rifampicin 60 mg/l in drinking water for 4 weeks. The group three mice received only saline as negative control. Bacterial load in spleen were determined for 8 weeks of infection at an interval of 10 days.
Accession numbers
Nucleotide sequences of L7/L12 (372 bp) and Omp25 (570 bp) from B. abortus S19 strain obtained from the sequencing of the PCR-amplified products were submitted to GenBank under the accession numbers MH568743 and MH568744 respectively.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Science (SPSS) version 16. Analysis of variance (ANOVA) at a confidence interval of 95% was used to analyze statistical difference exhibited by rL7/L12-Omp25 in comparison with S19 strain and PBS with respect to parameters including antibody titer, isotyping, and splenic count of B. abortus 544 virulent strain and differences were considered statistically significant at a p value ≤ 0.05. Data are expressed as the mean ± standard deviation.
Results
Construction, expression, and purification of L7/L12-Omp25 fusion molecule in E. coli
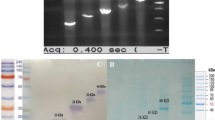
Successful PCR amplification of L7/L12 (372 bp) and Omp25 (570 bp) from B. abortus strain S19 was achieved using the designed set of primers. In order to create a fusion recombinant protein of L7/L12 and Omp25, the expression vector pRSET A was first constructed by cloning full length spliced via G4S as linker as described in the “Materials and methods” section. The recombinant plasmid, designated as pRSETL7/L12-Omp25, was verified by restriction digestion and in-frame insertion of both genes in the vector was confirmed by DNA sequencing. The transformed positive clone of E. coli BL21(DE3) pLysS was further optimized for expression of recombinant L7/L12-Omp25 (rL7/L12-Omp25) with induction time (4 h), IPTG concentration (1.0 mM), and growth temperature (37 °C). The recombinant protein expressed was found in inclusion bodies and purified by urea denaturation method. Further, the purified protein was refolded by subjecting it to gradient dialysis. The recombinant protein was detected with anti-His tag antibody by Western blot analysis (Fig. 1). The protein was approximately 41 kDa in size as witnessed in Western blot assay, corresponding to the predicted ORF. The protein concentration was determined by Bradford’s assay and stored in − 20 °C for further immunization and other experiments. The endotoxin level was found to be 0.02 pg of LPS equivalent/μg of rL7/L12-Omp25, within permissible limit of the European pharmacopeia current standard limit.
(A) Heterologous expression and purification of recombinant L7/L12-Omp25. SDS-PAGE showing expression of rL7/L12-Omp25. Lane UI: Lysate of uninduced E. coli BL21(DE3) pLysS clone; Lane I: induced E. coli BL21(DE3) pLysS L7/L12-Omp25 clone (1 mM IPTG), induced E. coli BL21(DE3) pLysS, Lane P: purified rL7/L12-Omp25 protein. (B) Western blot analysis of purified rL7/L12-Omp25. Lane 1: Protein marker; Lane 2: Purified rL7/L12-Omp25 protein
The inclusion of murine rIFN-γ for immunization enhances specific humoral response against the chimera
When evaluated for immunogenicity and type of immune response evoked, rL7/L12-Omp25 was found to elicit sufficient antibody response in mice 2 weeks after completion of immunization schedule. The specific antibodies against rL7/L12-Omp25 were observed in immunized mice with higher dilutions screened, but not in the pre-immunized sera (p < 0.05). The antibody response was similar to B. abortus S19 strain-immunized group until 15 days of immunization (ranging from 16,000 to 32,000). By 30 days of immunization, mice groups which received rL7/L12-Omp25 demonstrated higher titer of immunoglobulins (mean titer of 1,92,000) than that induced by S19 strain (mean titer of 32,000). The antigen-specific antibody titer was higher in the mice group immunized with cocktail of rL7/L12-Omp25 and rIFN-γ (raising up to a mean titer of 2,56,000) (Fig. 2a).
a Screening of rL7/L12-Omp25-specific IgG in immunized BALB/c mice. Mice were immunized with rL7/L12-Omp25 with or without rIFN-γ. Control group received only PBS. The sera were collected (n = 4) after immunization. The levels of rL7/L12-Omp25-specific IgG were analyzed by ELISA. The presence of specific antibodies to the recombinant protein was recorded in sera from both groups of L7/L12-Omp25-immunized mice but not in sham-immunized sera (p < 0.05). Data represent the mean absorbance (OD 450 nm) ± SD. b Analysis of rL7/L12-Omp25-specific polyclonal antibody isotypes in immunized BALB/c mice. The sera were collected from all four groups (n = 4 mice per group) after completion of immunization schedule. The levels of IgG subclasses and IgM were determined by ELISA. Data represent the mean absorbance (OD 450 nm) ± SD. The IgG2a/IgG1 ratio of rL7/L12-Omp25-immunized mice is 0.46, 0.81, and 0.65 from group 1 through 3. The error bar denotes the standard error
rL7/L12-Omp25 stimulates strong Th1-based immune response in mice
The results obtained in the ELISA-based isotyping experiment indicated significantly higher titers (p < 0.05) of IgG1, IgG2a, IgG2b, and IgG3 antibodies in rL7/L12-Omp25-immunized mice when compared with the titers recorded from the same mice before immunization (Fig. 2b). The predominant Ig subclass elicited among rL7/L12-Omp25-immunized mice (with or without rIFN-γ) was IgG1. The profile in the groups studied after 75 days of immunization schedule is tabulated in Table 1. The ratio of IgG2a/IgG1 in mice of groups immunized with rL7/L12-Omp25 with or without rIFN-γ was determined to be 0.46 and 0.81 respectively and the ratio in case of mice immunized with B. abortus S19 was 0.65.
The chimera induces antigen-specific memory response
We examined the cell stimulatory activity of rL7/L12-Omp25 on splenocytes of immunized mice as an indicator of induction of memory response by rL7/L12-Omp25. The SI of splenocytes from rL7/L12-Omp25-immunized mice from different immunization groups are indicated in Fig. 3. BALB/c mice from groups 1 and 2 (those received rL7/L12-Omp25 alone and rL7/L12-Omp25 + rIFN-γ respectively) demonstrated increase in proliferative activity; SI of group administered with rIFN-γ being more, compared to positive control, i.e., ConA-treated splenocytes. To explain, the stimulation index for splenocytes intraperitoneally immunized with rL7/L12-Omp25 alone was determined to be 2.0 (p = 0.018), whereas for that of rL7/L12-Omp25 + rIFN-γ splenocytes, it was found to be 3.02 (p = 0.099). Lymphocytes from sham-immunized mice showed no proliferative response against rL7/L12-Omp25. Interestingly, the group of rL7/L12-Omp25 + rIFN-γ-stimulated splenocytes registered higher proliferation rate when compared to splenocytes from S19-immunized mice (SI = 1.94, p = 0.0113). These high proliferation indices indicate that a specific clonal expansion of the splenocytes occurs in immunized BALB/c mice in response to rL7/L12-Omp25.
Lymphocyte proliferation assay. BALB/c mice were intraperitoneally immunized with rL7/L12-Omp25 alone, rL7/L12-Omp25 in cocktail with murine IFN-γ, and B. abortus S19 strain. On the 30th day post-immunization, splenic cells of mice were collected and stimulated with rL7/L12-Omp25 (10 μg/ml) for 72 h in triplicates. Concanavalin A was used as positive control at concentration of 3 μg/ml. Lymphocyte proliferation was estimated by MTT assay and absorbance was measured at 540 nm. The stimulation index (SI) of splenic cells within different groups was calculated to determine the proliferation activity. The error bar denotes the standard deviation
rL7/L12-Omp25 evokes early-stage and memory host response and resistance against Brucella infection
The cytokine secretion profile by T lymphocytes provides the evidence of polarization of CD4+ T cell response. T lymphocytes isolated from rL7/L12-Omp25-sensitized spleen were also evaluated in this line. Cytokine levels for IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-10, IL-12 (p70), and GM-CSF secreted by rL7/L12-Omp25-stimulated T lymphocytes are shown in Fig. 4. IFN-γ, TNF-α, and IL-10 showed significant increased level in mice groups immunized with rL7/L12-Omp25, rL7/L12-Omp25 + IFN-γ, and S19 strain. The level of IFN-γ was high in rL7/L12-Omp25 + IFN-γ-immunized mice (1833.24 pg/ml, p < 0.0001) than in rL7/L12-Omp25 (672.27 pg/ml, p < 0.0001) and S19 strain (1752.13 pg/ml, p < 0.0001) groups. On the other hand, rL7/L12-Omp25-immunized mice showed remarkable increase in levels of TNF-α (827 pg/ml, p = 0.0001). The levels of this pro-inflammatory cytokine were high in rL7/L12-Omp25 + IFN-γ (218.59 pg/ml, p = 0.001) and S19 strain (696.75, p = 0.001)-immunized mice as well. IL-10 production among the three groups was also high reaching 317.94 pg/ml (p = 0.0001), 1045.44 pg/ml (p = 0.0001), and 1584.69 pg/ml (p = 0.0001) respectively in groups of rL7/L12-Omp25, rL7/L12-Omp25 + IFN-γ, and S19 strain-immunized mice. But, the levels of IL-2, IL-4, IL-5, IL-12 (p70), and GM-CSF were noticeably low among splenocytes from all four mice groups.
rL7/L12-Omp25 induces IL-10, IFN-γ, and TNF-α from splenocytes. Comparative levels of individual cytokines produced by sensitized splenocytes from mice (n = 4) of all six immunization groups. All cells were sensitized with 10 μg/ml of rL7/L12-Omp25 for 72 h. Cytokine concentrations (pg/ml) were assayed in culture supernatants. Remarkable increase in production of IFN-γ, TNF-α, and IL-10 was observed among mice immunized with rL7/L12-Omp25. The error bar denotes the standard deviation
Immunization of BALB/c mice with rL7/L12-Omp25 elicits strong cell-mediated immune response
To assess the immune response elicited by rL7/L12-Omp25 among immunized mice, we also determined the range of T cell and B cell surface markers expression using whole blood samples (Figs. 5 and 6). Upon co-evaluation of lymphocytes for CD3 and CD45/B220 cell surface markers, CD3+ T cells were found to be in higher population (an average of 75% in group 1: rL7/L12-Omp25 immunized, 78.54% in group 2: rL7/L12-Omp25 + rIFN-γ immunized, 67.63% in group 3: S19 strain immunized, and 46.34% in group 4: sham immunized) than that of B cell-specific CD45/B220 (an average of 18.77%, 14.51%, 20.1%, and 39.1% from group 1 through group 4). On the other hand, in case of examining CD4 and CD8 cell surface markers, it was found that CD4+ T cells were predominantly expressed in all mice immunized with rL7/L12-Omp25 in presence (an average of 51.48%, p < 0.001) and absence of IFN-γ (an average of 45.15%, p < 0.05). The percentage of CD4+ T cells in case of B. abortus S19-immunized mice (an average of 41%, p < 0.001) was comparable with that of mice immunized with rL7/L12-Omp25 alone. No significant difference was observed in case of rate of CD8+ T cells in case of rL7/L12-Omp25-immunized mice and control mice group (an average of 16.82%, 14.24%, 15.83%, and 17.66% from group 1 through group 4). This clearly indicated that the total T cells were in higher population when compared to that of naïve mice, in turn indicating that the rL7/L12-Omp25-specific response was completely a strong cell-mediated biased response in vivo.
B cell subpopulation was estimated by flow cytometric analysis using fluorescent-tagged anti-CD45/B220 and anti-CD3 monoclonal antibodies. Blood from each mice group was collected 30 days post-immunization and used in the assay. Lymphocytes were prepared using blood and stained with FITC anti-mouse CD45/B220 antibody and PE anti-mouse CD3 antibody. The cell count acquisition for the frequency of CD3+CD45/B220+ was performed by two-color fluorescence flow cytometric analysis by a BD FACS Calibur system. The results were analyzed using FlowJo v.10.1 software
T cell subpopulation was estimated by flow cytometric analysis using fluorescent-tagged anti-CD4 and anti-CD8 monoclonal antibodies. Blood from each mice group was collected 30 days post-immunization and used in the assay. Lymphocytes were prepared using blood and stained with FITC anti-mouse CD4 antibody and PE anti-mouse CD8a antibody. The cell count acquisition for the frequency of CD4+CD8+ was performed by two-color fluorescence flow cytometric analysis by a BD FACS Calibur system. The results were analyzed using FlowJo v.10.1 software
Vaccination of rL7/L12-Omp25 confers protective immunity against B. abortus strain 544 challenge by reducing bacterial burden
In order to determine whether intraperitoneal immunization of BALB/c mice with rL7/L12-Omp25 was capable of conferring protection against infection challenge with B. abortus strain 544, all challenged mice were observed periodically and euthanized after 8 weeks of challenge. Sham-immunized mice were found to present high splenic count of B. abortus within 8 weeks post challenge. On the other hand, minimal bacterial burden was noticed in rL7/L12-Omp25-immunized mice in that time frame (Table 2). The log protection units registered in mice immunized with rL7/L12-Omp25 alone (2.16, p = 0.0001) and in cocktail with murine IFN-γ (2.4, p = 0.0001) were superior to that of B. abortus strain S19-immunized mice (1.79, p = 0.0001). Among rL7/L12-Omp25 alone and rL7/L12-Omp25 + IFN-γ-immunized mice, no significant difference was observed but both groups showed significant difference in comparison with sham-immunized mice group.
Therapeutic efficacy of rL7/L12-Omp25 and IFN-γ cocktail
Mice infected with B. abortus 544 strain were treated with either cocktail of rL7/L12-Omp25 and IFN-γ or rifampicin, and the therapeutic efficacy was determined by splenic count. A significant reduction in the bacterial burden among mice treated with rifampicin was recorded; however, treatment with rL7/L12-Omp25 and IFN-γ was not effective in clearing bacterial load in mice spleen (Fig. 7).
Assessment of therapeutic effect of rL7/L12-Omp25 protein on B. abortus 544 strain infected BALB/c mice. Bacterial load from rifampicin- and rL7/L12-Omp25 + rIFN-γ-treated mice was determined for 8 weeks of infection at an interval of 10 days. The bacterial burden found to significantly increase in case of rL7/L12-Omp25 + rIFN-γ-treated mice indicated the failure of the molecule to induce therapeutic effect in bacterial clearance. On the other hand, gradual decrease in CFU was observed in case of rifampicin-treated mice indicating its therapeutic effect on Brucella infection
Discussion
A new generation Brucella vaccine is expected to countermine the major drawbacks such as abortion in pregnant animals, infection in human hosts, and interference with pathogenic strains in diagnosis, which the presently recommended attenuated vaccine strains manifest (Moriyón et al. 2004). Advent of subunit vaccines comprising of purified recombinant proteins have embarked promise as an alternate, safer, and reliable solution. However, care must be taken to select appropriate immunogens that confer the required strong CMI response against brucellosis comparable to that of attenuated Brucella vaccines in use (Zhan et al. 1993). Co-formulation of an efficacious candidate vaccine with suitable key molecule that could be instrumental in enduring the required type of immunity is another approach that merits an attempt to be trialed in Brucella vaccines. Cytokines are one such group of molecules that play major role in promoting immunity against Brucella. Among them, IFN-γ is proven to upregulate anti-Brucella activity among macrophages and effect delayed-type inflammatory (Baldwin and Winter 1994; Murphy et al. 2001). Earlier studies have demonstrated that co-immunization of a given antigen along with IFN-γ induces strongly enhanced proliferation of T cells and increased production of IFN-γ in response to the antigen (Hovav et al. 2005). Thus, to take full advantage of IFN-γ, we conceptualized a co-formulation of this cytokine with suitable chimeric polyvalent vaccine that can induce increased immune response than a monovalent vaccine in our design.
Several Brucella proteins have been investigated as antigens with potential to impart effective immunity against brucellosis. Among them, L7/L12 protein has shown to elicit immune response in murine model when administered in free form by activating both B and T cells (Oliveira and Splitter 1994, 1996; Mallick et al. 2007). Brucella Omp25 is another antigen, a major Brucella outer membrane protein that has been shown to be an immunogen and a virulent factor in Brucella infection (Goel and Bhatnagar 2012; Edmonds et al. 2001; Edmonds et al. 2002a, b). Protection conferred by anti-Omp25 antibodies in mice upon passive immunization confirms the importance of this immunogen as a promising vaccine candidate (Bowden et al. 1995a, b). Commander and co-workers reported that Omp25 conferred protection against B. melitensis when administered to BALB/c mice as a DNA vaccine candidate (Commander et al. 2007). Thus, L7/L12 and Omp25 were chosen as suitable immunogens to make divalent Brucella vaccine to achieve desirable protective immune responses. In this study, our primary aim was to develop a chimeric fusion protein comprising of L7/L12 and Omp25 from Brucella and to investigate its vaccine efficacy in silico, in vitro, and in vivo experimental trials.
Immunization of BALB/c mice with rL7/L12-Omp25 generated high antibody titer as revealed by ELISA as compared to S19 vaccine. However, rL7/L12-Omp25 antibody titer was superior to that of S19 strain, when administered along with murine IFN-γ. Total IgG level was observed to significantly raise at various time points. This suggests that intraperitoneal immunization of rL7/L12-Omp25 in cocktail form with murine IFN-γ induces B cell activation for the production of promising antibodies.
We assessed titers of IgG1 and IgG2a antibody isotypes generated in the immunized mice against rL7/L12-Omp25 protein to evaluate help T cell response. Significantly, higher level of IgG1 and IgG2a was determined in the sera collected from mice immunized with rL7/L12-Omp25 in presence and absence of murine IFN-γ. In fact, a continuous higher IgG1 level was maintained in the animals immunized with rL7/L12-Omp25, as well as S19 vaccine strain, as compared to its IgG2a counterpart. Similar observation of predominant induction of IgG1 class antibodies is studied among calves vaccinated with Brucella abortus S19 and RB51 indicating a strong and complex Th2-based response (Dorneles et al. 2015).
The role of recombinant rL7/L12-Omp25 in elicitation of cell-mediated immune response among immunized mice was evaluated by measuring T cell proliferation response by stimulating splenic cells in vitro with purified rL7/L12-Omp25. Evidently, immunization of mice with rL7/L12-Omp25 and murine IFN-γ resulted in significantly higher splenic proliferation rate comparable to that resulting from immunization with B. abortus S19. Our results are indicative of the promising efficacy of rL7/L12-Omp25 in induction of CD4+ T lymphocyte-mediated immune response which plays a key role in bactericidal activity by activating macrophages as reported in other similar studies (Fernandes and Baldwin 1995; Baldwin and Winter 1994; Olsen 2013).
Upregulated secretion of two pro-inflammatory cytokines namely TNF-α and IFN-γ by rL7/L12-Omp25-specific T lymphocytes demonstrated the induction of strong CMI among immunized mice. Production of TNF-α and IFN-γ could be a reason for successful bacterial clearance from infected mice observed, since these molecules are well documented for clearance of facultative intracellular pathogens (Baldwin and Winter 1994; Zhan et al. 1996). Strong IFN-γ response and predominance of IgG1 antibodies in the rL7/L12-Omp25 immunized mice (with or without murine IFN-γ) suggested possible elicitation of both Th1 and Th2 type of immune response against the antigen in the host. The production of this pro-inflammatory mediator, i.e., IFN-γ, reflected the type 1 T helper (Th1)-polarized response that would result in macrophage activation to contribute host resistance against intracellular infection (Ulett et al. 2000). The critical role of IFN-γ in resolving brucellosis has been reported in several studies (Fernandes and Baldwin 1995; Olsen 2013). Increased secretion of IFN-γ among rL7/L12-Omp25-immunized mice could be a contribution of L7/L12 component and TNF-α by Omp25 component as reported in previous studies (Kazak et al. 2010; Ma et al. 2015). Other cytokines were induced at very low levels. Elevated production of IL-10 is a proven mechanism associated with Brucella infection (Fernandes et al. 1996). Both anti-inflammatory and pro-inflammatory cytokines are involved in the formation of well-differentiated, bactericidal macrophages that play an essential role in preventing the proliferation and dissemination of intracellular pathogens. Our study suggests that L7/L12 and Omp25 of Brucella may be involved in inducing IL-10 production as a survival mechanism. In this study, inclusion of murine IFN-γ for immunizing mice with rL7/L12-Omp25 showed negligible changes of pattern of immune response elicitation when compared to rL7/L12-Omp25 alone. The unmodified levels of IL-4 among all four groups of mice studies indicated no involvement of this Th2 representative cytokine in the immune response against brucellosis, a common observation reported in earlier studies as well (Pasquali et al. 2001; Rodríguez-Zapata et al. 2010). Quantification of CD markers on cell surface of lymphocytes from mice groups also augmented the observation that rL7/L12-Omp25 induced a strong Th1-biased immune response and this response evidently increased with the inclusion of murine rIFN-γ during immunization.
To examine the vaccine potential of rL7/L12-Omp25, protection studies were performed and evaluated in terms of splenic bacterial burden of immunized mice. Groups of mice vaccinated with rL7/L12-Omp25 antigen (with or without rIFN-γ) showed remarkably higher protection at each time point than other control groups. It was found that bacterial load determined on day 30 post-infection showed superior level of protection efficacy of rL7/L12-Omp25 against Brucella infection when compared to S19 vaccine strain. However, the molecule failed to register a promising therapeutic effect against brucellosis as it failed to reduce the bacterial burden in spleens among infected mice. The reason for this failure of rL7/L12-Omp25 to embark therapeutic effect in spite of being a superior prophylactic molecule needs to be deciphered with further experimental studies.
To conclude, this is the first report of using L7/L12 as a divalent antigen along with the Omp25, a proven potent vaccine candidate, along with murine IFN-γ intraperitoneally in murine model as a cocktailed subunit vaccine against brucellosis. The results obtained in this study explored the possibility of using the cocktailed subunit vaccine formulation in order to achieve higher protection levels to the par of Brucella live vaccines.
References
Al-Mariri A, Tibor A, Mertens P, De Bolle X, Michel P, Godefroid J, Walravens K, Letesson JJ (2001) Protection of BALB/c mice against Brucella abortus 544 challenge by vaccination with bacterioferritin or P39 recombinant proteins with CpG oligodeoxy nucleotides as adjuvant. Infect Immun 69(8):4816–4822. https://doi.org/10.1128/IAI.69.8.4816-4822.2001
Araya LN, Elzer PH, Rowe GE, Enright FM, Winter AJ (1989) Temporal development of protective cell-mediated and humoral immunity in BALB/c mice infected with Brucella abortus. J Immunol 143(10):3330–3337
Baldwin CL, Winter AJ (1994) Macrophages and Brucella. Immunol Ser 60:363–380
Baloglu S, Toth TE, Schurig GG, Sriranganathan N, Boyle SM (2000) Humoral immune response of BALB/c mice to a vaccinia virus recombinant expressing Brucella abortus GroEL does not correlate with protection against a B. abortus challenge. Vet Microbiol 76(2):193–199. https://doi.org/10.1016/S0378-1135(00)00231-5
Bowden RA, Cloeckaert A, Zygmunt MS, Bernard S, Dubray G (1995a) Surface exposure of outer membrane protein and lipopolysaccharide epitopes in Brucella species studied by enzyme-linked immunosorbent assay and flow cytometry. Infect Immun 63(10):3945–3952
Bowden RA, Cloeckaert A, Zygmunt MS, Dubray G (1995b) Outer-membrane protein-and rough lipopolysaccharide-specific monoclonal antibodies protect mice against Brucella ovis. J Med Microbiol 43(5):344–347. https://doi.org/10.1099/00222615-43-5-344
Cassataro J, Velikovsky CA, de la Barrera S, Estein SM, Bruno L, Bowden R, Pasquevich KA, Fossati CA, Giambartolomei GH (2005) A DNA vaccine coding for the Brucella outer membrane protein 31 confers protection against B. melitensis and B. ovis infection by eliciting a specific cytotoxic response. Infect Immun 73(10):6537–6546
Cassataro J, Pasquevich KA, Estein SM, Laplagne DA, Zwerdling A, de la Barrera S, Bowden R, Fossati CA, Giambartolomei GH, Goldbaum FA (2007) A DNA vaccine coding for the chimera BLS Omp31 induced a better degree of protection against B. ovis and a similar degree of protection against B. melitensis than Rev.1 vaccination. Vaccine 25(32):5958–5967
Commander NJ, Spencer SA, Wren BW, MacMillan AP (2007) The identification of two protective DNA vaccines from a panel of five plasmid constructs encoding Brucella melitensis 16 M genes. Vaccine 25(1):43–54. https://doi.org/10.1016/j.vaccine.2006.07.046
De Fays K, Tibor A, Lambert C, Vinals C, Denoel P, De Bolle X, Wouters J, Letesson JJ, Depiereux E (1999) Structure and function prediction of the Brucella abortus P39 protein by comparative modeling with marginal sequence similarities. Protein Eng 12(3):217–223. https://doi.org/10.1093/protein/12.3.217
Delpino MV, Estein SM, Fossati CA, Baldi PC, Cassataro J (2007) Vaccination with Brucella recombinant DnaK and SurA proteins induces protection against Brucella abortus infection in BALB/c mice. Vaccine 25(37):6721–6729
Denoel PA, Vo TK, Tibor A, Weynants VE, Trunde JM, Dubray G, Limet JN, Letesson JJ (1997) Characterization, occurrence, and molecular cloning of a 39-kilodalton Brucella abortus cytoplasmic protein immunodominant in cattle. Infect Immun 65(2):495–502
Dorneles EM, Lima GK, Teixeira-Carvalho A, Araújo MS, Martins-Filho OA, Sriranganathan N, Al Qublan H, Heinemann MB, Lage AP (2015) Immune response of calves vaccinated with Brucella abortus S19 or RB51 and revaccinated with RB51. PLoS One 10(9):e0136696. https://doi.org/10.1371/journal.pone.0136696
Edmonds MD, Cloeckaert A, Booth NJ, Fulton WT, Hagius SD, Walker JV, Elzer PH (2001) Attenuation of a Brucella abortus mutant lacking a major 25 kDa outer membrane protein in cattle. Am J Vet Res 62(9):1461–1466. https://doi.org/10.2460/ajvr.2001.62.1461
Edmonds MD, Cloeckaert A, Elzer PH (2002a) Brucella species lacking the major outer membrane protein Omp25 are attenuated in mice and protect against Brucella melitensis and Brucella ovis. Vet Microbiol 88(3):205–221. https://doi.org/10.1016/S0378-1135(02)00110-4
Edmonds MD, Cloeckaert A, Hagius SD, Samartino LE, Fulton WT, Walker JV, Enright FM, Booth NJ, Elzer PH (2002b) Pathogenicity and protective activity in pregnant goats of a Brucella melitensis Delta omp25 deletion mutant. Res Vet Sci 72(3):235–239. https://doi.org/10.1053/rvsc.2002.0555
Estein SM, Cassataro J, Vizcaíno N, Zygmunt MS, Cloeckaert A, Bowden RA (2003) The recombinant Omp31 from Brucella melitensis alone or associated with rough lipopolysaccharide induces protection against Brucella ovis infection in BALB/c mice. Microbes Infect 5(2):85–93. https://doi.org/10.1016/S1286-4579(02)00075-8
Fernandes DM, Baldwin CL (1995) Interleukin-10 down regulates protective immunity to Brucella abortus. Infect Immun 63(3):1130–1133
Fernandes DM, Jiang X, Jung JH, Baldwin CL (1996) Comparison of T cell cytokines in resistant and susceptible mice infected with virulent Brucella abortus strain 2308. FEMS Immunol Med Microbiol 16(3–4):193–203. https://doi.org/10.1111/j.1574-695X.1996.tb00136.x
Goel D, Bhatnagar R (2012) Intradermal immunization with outer membrane protein 25 protects Balb/c mice from virulent B. abortus 544. Mol Immunol 51(2):159–168. https://doi.org/10.1016/j.molimm.2012.02.126
Hovav AH, Fishman Y, Bercovier H (2005) Gamma interferon and monophosphoryl lipid A-trehalosedicorynomycolate are efficient adjuvants for Mycobacterium tuberculosis multivalent acellular vaccine. Infect Immun 73(1):250–257
Kazak E, Oliveira SC, Goral G, Akalin H, Yilmaz E, Heper Y, Oral HB (2010) Brucella abortus L7/L12 recombinant protein induces strong Th1 response in acute brucellosis patients. Iran J Immunol 7(3):132–141
Kurar E, Splitter GA (1997) Nucleic acid vaccination of Brucella abortus ribosomal L7/L12 gene elicits immune response. Vaccine 15(17–18):1851–1857. https://doi.org/10.1016/S0264-410X(97)00140-0
Lin J, Adams LG, Ficht TA (1996) Immunological response to the Brucella abortus GroEL homolog. Infect Immun 64(10):4396–4400
Luo D, Ni B, Li P, Shi W, Zhang S, Han Y, Mao L, He Y, Wu Y, Wang X (2006) Protective immunity elicited by a divalent DNA vaccine encoding both the L7/L12 and Omp16 genes of Brucella abortus in BALB/c mice. Infect Immun 74(5):2734–2741. https://doi.org/10.1128/iai.74.5.2734-2741.2006
Ma QL, Liu AC, Ma XJ, Wang YB, Hou YT, Wang ZH (2015) Brucella outer membrane protein Omp25 induces microglial cells in vitro to secrete inflammatory cytokines and inhibit apoptosis. Int J Clin Exp Med 8(10):17530–17535
Mallick AI, Singha H, Chaudhuri P, Nadeem A, Khan SA, Dar KA, Owais M (2007) Liposomised recombinant ribosomal L7/L12 protein protects BALB/c mice against Brucella abortus 544 infection. Vaccine 25(18):3692–3704. https://doi.org/10.1016/j.vaccine.2007.01.066
Moriyón I, Grilló MJ, Monreal D, González D, Marín C, López-Goñi I, Mainar-Jaime RC, Moreno E, Blasco JM (2004) Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet Res 35(1):1–38. https://doi.org/10.1051/vetres:2003037
Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL (2001) Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103(4):511–518. https://doi.org/10.1046/j.1365-2567.2001.01258.x
O’Garra A, Murphy K (1994) Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol 6(3):458–466. https://doi.org/10.1016/0952-7915(94)90128-7
Oliveira SC, Splitter GA (1994) Subcloning and expression of the Brucella abortus L7/L12 ribosomal gene and T-lymphocyte recognition of the recombinant protein. Infect Immun 62(11):5201–5204
Oliveira SC, Splitter GA (1996) Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine 14(10):959–962
Oliveira SC, Zhu Y, Splitter GA (1994) Recombinant L7/L12 ribosomal protein and gamma-irradiated Brucella abortus induce a T-helper 1 subset response from murine CD4+ T cells. Immunology 83(4):659–664
Olsen SC (2013) Recent developments in livestock and wildlife brucellosis vaccination. Rev Sci Tech 32(1):207–217. https://doi.org/10.1007/s11515-012-1196-0
Pasquali P, Adone R, Gasbarre LC, Pistoia C, Ciuchini F (2001) Mouse cytokine profiles associated with Brucella abortus RB51 vaccination or B. abortus 2308 infection. Infect Immun 69(10):6541–6544. https://doi.org/10.1128/IAI.69.10.6541-6544.2001
Rodríguez-Zapata M, Matías MJ, Prieto A, Jonde MA, Monserrat J, Sánchez L, Reyes E, De la Hera A, Alvarez-Mon M (2010) Human brucellosis is characterized by an intense Th1 profile associated with a defective monocyte function. Infect Immun 78(7):3272–3279. https://doi.org/10.1128/IAI.01385-09
Schurig GG, Sriranganathan N, Corbel MJ (2002) Brucellosis vaccines: past, present and future. Vet Microbiol 90(1–4):479–496
Stevens MG, Pugh GW, Tabatabai LB (1992) Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect Immun 60(10):4407–4409
Tabatabai LB, Pugh GW (1994) Modulation of immune responses in BALB/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine 12(10):919–924
Tadepalli G, Singh AK, Konduru B, Murali HS, Batra HV (2016) Immunogenicity and protective efficacy of Brucella abortus recombinant protein cocktail (rOmp19 + rP39) against B. abortus 544 and B. melitensis 16M infection in murine model. Mol Immunol 71:34–41
Thomson CA, Olson M, Jackson LM, Schrader JW (2012) A simplified method for the efficient refolding and purification of recombinant human GM-CSF. PLoS One 7(11):e49891. https://doi.org/10.1371/journal.pone.0049891
Toth TE, Cobb JA, Boyle SM, Roop RM, Schurig GG (1995) Selective humoral immune response of BALB/c mice to Brucella abortus proteins expressed by vaccinia virus recombinants. Vet Microbiol 45(2–3):171–183
Ulett GC, Ketheesan N, Hirst RG (2000) Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect Immun 68(4):2034–2042
Zhan Y, Cheers C (1993) Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect Immun 61(11):4899–4901
Zhan Y, Yang J, Cheers C (1993) Cytokine response of T-cell subsets from Brucella abortus-infected mice to soluble Brucella proteins. Infect Immun 61(7):2841–2847
Zhan Y, Liu Z, Cheers C (1996) Tumor necrosis factor alpha and interleukin-12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect Immun 64(7):2782–2786
Acknowledgments
The first and second authors are thankful to the Indian Council of Medical Research (ICMR), India, and the Department of Science and Technology (DST), India, for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Paul, S., Peddayelachagiri, B.V., Nagaraj, S. et al. Protective and therapeutic efficacy study of divalent fusion protein rL7/L12-Omp25 against B. abortus 544 in presence of IFNγ. Appl Microbiol Biotechnol 102, 8895–8907 (2018). https://doi.org/10.1007/s00253-018-9314-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9314-9