Abstract
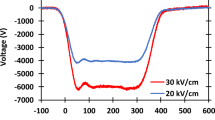
Antibiotic resistance mechanisms render current antibiotics ineffective, requiring higher concentrations of existing drugs or the development of more powerful drugs for infection treatment. This study demonstrates the synergistic inactivation of a gram-positive (Staphylococcus aureus) and a gram-negative (Escherichia coli) bacteria by combining either tobramycin or rifampicin with 300-ns electric pulses (EPs). For EPs depositing the same total energy density into the sample with no drug, higher electric fields induced greater inactivation, indicating a threshold for irreversible electroporation at these fields and membrane recovery in between lower intensity EPs. Synergistic inactivation generally increased with increasing drug concentration up to 20 μg/mL compared to strictly EP treatment. Combining even 1/20 of the clinical dose of tobramycin with a train of EPs induced between 2.5 and 3.5 log inactivation after only 10 min of exposure compared to hours to induce inactivation with a clinical dose with no EPs. Similarly, combining a train of EPs with a clinically relevant dose of rifampicin induced 7 to 9 log inactivation over the same time of exposure. These results indicate the promise of combining EPs with antibiotics to rapidly inactivate antibiotic-resistant bacteria in localized treatment areas.





Similar content being viewed by others
References
Barba FJ, Parniakov O, Pereira SA, Wiktor A, Grimi N, Boussetta N, Saraiva JA, Raso J, Martin-Belloso O, Witrowa-Rajchert D (2015) Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res Int 77:773–798
Bassegoda A, Ivanova K, Ramon E, Tzanov T (2018) Strategies to prevent the occurrence of resistance against antibiotics by using advanced materials. Appl Microbiol Biotechnol 102:2075–2089
Blaser MJ (2014) Missing microbes: how the overuse of antibiotics is fueling our modern plagues. Macmillan, Basingstoke
Calvori C, Frontali L, Leoni L, Tecce G (1965) Effect of rifamycin on protein synthesis. Nature 207:417–418
Center for Disease Control (2013) Antibiotic resistance threats in the United States (2013). US Dep Health Hum Serv CDC, Atlanta
Chalise PR, Perni S, Shama G, Novac BM, Smith IR, Kong MG (2006) Lethality mechanisms in Escherichia coli induced by intense sub-microsecond electrical pulses. Appl Phys Lett 89:153902
Davies J (1996) Bacteria on the rampage. Nature 383:219–220
Del Pozo JL, Rouse MS, Patel R (2008) Bioelectric effect and bacterial biofilms. A systematic review. Int J Artif Organs 31:786–795
Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B (2006) In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng 53:1409–1415
Fleming AE, Chain EB, Florey H (1945) Nobel lecture on penicillin. Nobel Foundation Nobel Media AB https://www.nobelprize.org/nobel_prizes/medicine/laureates/1945/fleming-speech.html, Accessed 09 September 2017
Garner AL, Deminsky M, Bogdan Neculaes V, Chashihin V, Knizhnik A, Potapkin B (2013) Cell membrane thermal gradients induced by electromagnetic fields. J Appl Phys 113(21):214701
Garner AL, Caiafa A, Jiang Y, Klopman S, Morton C, Torres AS, Loveless AM, Neculaes VB (2017) Experimental validation of a compact, flexible pulsed power architecture for ex vivo platelet activation. PLoS One 12:e0181214
van Geelen L, Meier D, Rehberg N, Kalscheuer R (2018) Some current concepts in antibacterial drug discovery. Appl Microbiol Biotechnol https://doi.org/10.1007/s00253-018-8843-6, 102, 2949, 2963
Hamilton WA, Sale AJH (1967) Effects of high electric fields on microorganisms: II. Mechanism of action of the lethal effect. Biochim Biophys Acta BBA-Gen Subj 148:789–800
Hofmann GA, Dev SB, Dimmer SC, Levatter JI, Nanda GS (2000) Apparatus for addressing needle array electrodes for electroporation therapy U.S. Patent No 6,055,453
Jiang C, Davalos RV, Bischof JC (2015) A review of basic to clinical studies of irreversible electroporation therapy. IEEE Trans Biomed Eng 62:4–20
Jones MB, Nierman WC, Shan Y, Frank BC, Spoering A, Ling L, Peoples A, Zullo A, Lewis K, Nelson KE (2017) Reducing the bottleneck in discovery of novel antibiotics. Microb Ecol 73:658–667
Joshi RP, Schoenbach KH (2010) Bioelectric effects of intense ultrashort pulses. Crit Rev Biomed Eng 38:255–304
Kotra LP, Haddad J, Mobashery S (2000) Aminoglycosides: perspectives on mechanisms of action and resistance and strategies to counter resistance. Antimicrob Agents Chemother 44:3249–3256
Lambricht L, Lopes A, Kos S, Sersa G, Préat V, Vandermeulen G (2016) Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin Drug Deliv 13:295–310
Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10:S122–S129
Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, Mueller A, Schäberle TF, Hughes DE, Epstein S (2015) A new antibiotic kills pathogens without detectable resistance. Nature 517:455–459
Madigan MT, Martinko JM, Parker J, Brock TD (1997) Brock biology of microorganisms. Prentice hall Upper Saddle River, NJ
Miklavčič D, Mali B, Kos B, Heller R, Serša G (2014) Electrochemotherapy: from the drawing board into medical practice. Biomed Eng Online 13:29
Nordmann P, Poirel L, Toleman MA, Walsh TR (2011) Does broad-spectrum β-lactam resistance due to NDM-1 herald the end of the antibiotic era for treatment of infections caused by gram-negative bacteria? J Antimicrob Chemother 66:689–692
Nuccitelli R, Wood R, Kreis M, Athos B, Huynh J, Lui K, Nuccitelli P, Epstein EH Jr (2014) First-in-human trial of nanoelectroablation therapy for basal cell carcinoma: proof of method. Exp Dermatol 23:135–137
Perni S, Chalise PR, Shama G, Kong MG (2007) Bacterial cells exposed to nanosecond pulsed electric fields show lethal and sublethal effects. Int J Food Microbiol 120:311–314
Piddock LJ (2006) Multidrug-resistance efflux pumps–not just for resistance. Nat Rev Microbiol 4:629–636
Reardon S (2014) Antibiotic resistance sweeping developing world: bacteria are increasingly dodging extermination as drug availability outpaces regulation. Nature 509:141–143
Reynolds R, Potz N, Colman M, Williams A, Livermore D, MacGowan A (2004) Antimicrobial susceptibility of the pathogens of bacteraemia in the UK and Ireland 2001–2002: the BSAC Bacteraemia Resistance Surveillance Programme. J Antimicrob Chemother 53:1018–1032
Roth CC, Barnes RA Jr, Ibey BL, Beier HT, Mimun LC, Maswadi SM, Shadaram M, Glickman RD (2015) Characterization of pressure transients generated by nanosecond electrical pulse (nsEP) exposure. Sci Rep 5:15063
Sale AJH, Hamilton WA (1967) Effects of high electric fields on microorganisms: I. Killing of bacteria and yeasts. Biochim Biophys Acta BBA-Gen Subj 148:781–788
Sale AJH, Hamilton WA (1968) Effects of high electric fields on micro-organisms: III Lysis of erythrocytes and protoplasts. Biochim Biophys Acta BBA-Biomembr 163:37–43
Schoenbach KH, Peterkin FE, Alden RW III, Beebe SJ (1997) The effect of pulsed electric fields on biological cells: experiments and applications. IEEE Trans Plasma Sci 25:284–292
Schoenbach KH, Xiao S, Joshi RP, Camp JT, Heeren T, Kolb JF, Beebe SJ (2008) The effect of intense subnanosecond electrical pulses on biological cells. IEEE Trans Plasma Sci 36:414–422
Schoenbach KH, Joshi RP, Beebe SJ, Baum CE (2009) A scaling law for membrane permeabilization with nanopulses. Dielectr Electr Insul IEEE Trans On 16:1224–1235
Schoenbach KH, Pakhomov AG, Semenov I, Xiao S, Pakhomova ON, Ibey BL (2015) Ion transport into cells exposed to monopolar and bipolar nanosecond pulses. Bioelectrochemistry 103:44–51
Song J, Garner AL, Joshi RP (2017) Effect of thermal gradients created by electromagnetic fields on cell-membrane electroporation probed by molecular-dynamics simulations. Phys Rev Appl 7:024003
Vernier PT, Ziegler MJ, Sun Y, Chang WV, Gundersen MA, Tieleman DP (2006) Nanopore formation and phosphatidylserine externalization in a phospholipid bilayer at high transmembrane potential. J Am Chem Soc 128:6288–6289
Witte W (1998) Medical consequences of antibiotic use in agriculture. Science 279:996–997
World Health Organization (2014) Antimicrobial resistance: global report on surveillance. World Health Organization
Acknowledgments
We gratefully acknowledge Matt Hedrick for useful discussions.
Funding
This work was supported by the US Nuclear Regulatory Commission Nuclear Education Program Faculty Development Grant Program at Purdue University [grant number NRC-HQ-84-14-G-0048]. Nanovis, Inc. provided materials.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Vadlamani, A., Detwiler, D.A., Dhanabal, A. et al. Synergistic bacterial inactivation by combining antibiotics with nanosecond electric pulses. Appl Microbiol Biotechnol 102, 7589–7596 (2018). https://doi.org/10.1007/s00253-018-9215-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9215-y