Abstract
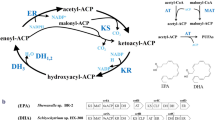
Thraustochytrium sp. 26185, a unicellular marine protist, synthesizes docosahexaenoic acid, an omega-3 very long chain polyunsaturated fatty acid (VLC-PUFAs), by a polyunsaturated fatty acid (PUFA) synthase comprising three large subunits with multiple catalytic dehydratase (DH) domains critical for introducing double bonds at the specific position of fatty acids. To investigate functions of these DH domains, one DH domain from subunit-A and two DH domains from subunit-C of the PUFA synthase were dissected and expressed as stand-alone enzymes in Escherichia coli. The results showed that all these DH domains could complement the defective phenotype of a E. coli FabA temperature sensitive mutant, despite they have only modest sequence similarity with FabA, indicating they can function as 3-hydroxyacyl-ACP dehydratase for the biosynthesis of unsaturated fatty acids in E. coli. Site-directed mutagenesis analysis confirmed the authenticity of active site residues in these domains. In addition, overexpression of the three domains in a wild type E. coli strain resulted in the substantial alteration of fatty acid profiles including productions and ratio of unsaturated to saturated fatty acids. A combination of evidences from sequence comparison, functional expression, and mutagenesis analysis suggest that the DH domain from subunit-A is similar to DH domains from polyketide synthases, while the DH domains from subunit-C are more comparable to E. coli FabA in catalytic functions. Successful complementation and functional expression of the embedded DH domains from the PUFA synthase in E. coli is an important step towards for elucidating the molecular mechanism in the biosynthesis of VLC-PUFAs in Thraustochytrium.






Similar content being viewed by others
References
Amiri-Jami M, Lapointe G, Griffiths MW (2014) Engineering of EPA/DHA omega-3 fatty acid production by Lactococcus lactis subsp. cremoris MG1363. Appl Microbiol Biotechnol 98(7):3071–3080. https://doi.org/10.1007/s00253-013-5381-0
Ansari MZ, Yadav G, Gokhale RS, Mohanty D (2004) NRPS-PKS: a knowledge-based resource for analysis of NRPS/PKS megasynthases. Nucleic Acids Res 32(Web Server):W405–W413. https://doi.org/10.1093/nar/gkh359
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Cassarino TG, Bertoni M, Bordoli L, Schwede T (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42(W1):W252–W258. https://doi.org/10.1093/nar/gku340
Chung CT, Niemela SL, Miller RH (1989) One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution (recombinant DNA). Biochemistry 86(7):2172–2175. https://doi.org/10.1073/pnas.86.7.2172
Conway KR, Boddy CN (2013) ClusterMine360: a database of microbial PKS/NRPS biosynthesis. Nucleic Acids Res 41(D1):D402–D407. https://doi.org/10.1093/nar/gks993
Cronan JE, Thomas J (2009) Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzym 459:395–433. https://doi.org/10.1016/S0076-6879(09)04617-5
Diau GY, Hsieh AT, Sarkadi NEA, Wijendran V, Nathanielsz PW, Brenna JT (2005) The influence of long chain polyunsaturate supplementation on docosahexaenoic acid and arachidonic acid in baboon neonate central nervous system. BMC Med 3(1):11. https://doi.org/10.1186/1741-7015-3-11
Feng Y, Cronan JE (2009) Escherichia coli unsaturated fatty acid synthesis: complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J Biol Chem 284(43):29526–29535. https://doi.org/10.1074/jbc.M109.023440
Finzel K, Nguyen C, Jackson DR, Gupta A, Tsai SC, Burkart MD (2015) Probing the substrate specificity and protein-protein interactions of the E. coli fatty acid dehydratase, FabA. Chem Biol 22(11):1453–1460. https://doi.org/10.1016/j.chembiol.2015.09.009
Gong Y, Wan X, Jiang M, Hu C, Hu H, Huang F (2014) Metabolic engineering of microorganisms to produce omega-3 very long-chain polyunsaturated fatty acids. Prog Lipid Res 56:19–35. https://doi.org/10.1016/j.plipres.2014.07.001
Hadders-Algra M (2010) Effect of long-chain polyunsaturated fatty acid supplementation on neurodevelopmental outcome in full-term infants. Nutrients 2(8):790–804. https://doi.org/10.3390/nu2080790
Heath RJ, Rock CO (1996) Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J Biol Chem 271(44):27795–27801. https://doi.org/10.1074/jbc.271.44.27795
Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77(1):51–59. https://doi.org/10.1016/0378-1119(89)90358-2
Horrocks LA, Yeo YK (1999) Health benefits of docosahexaenoic acid (DHA). Pharmacol Res 40(3):211–225. https://doi.org/10.1006/phrs.1999.0495
Ira H (1987) Functional inactivation of genes by dominant negative mutations. Nature 329(6136):219–222. https://doi.org/10.1038/329219a0
Keatinge-Clay AT (2012) The structures of type I polyketide synthases. Nat Prod Rep 29(10):1050–1073. https://doi.org/10.1039/c2np20019h
Kelley LA, Sternberg MJE (2009) Protein structure prediction on the web: a case study using the Phyre server. Nat Protoc 4(3):363–371. https://doi.org/10.1038/nprot.2009.2
Leesong M, Henderson BS, Gillig JR, Schwab JM, Smith JL (1996) Structure of a dehydratase–isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: two catalytic activities in one active site. Structure 4(3):253–264. https://doi.org/10.1016/S0969-2126(96)00030-5
Lu YJ, Zhang YM, Rock CO (2004) Product diversity and regulation of type II fatty acid synthases. Biochem Cell Biol 82(1):145–155. https://doi.org/10.1139/o03-076
Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R (2011) antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res 39(suppl_2):W339–W346. https://doi.org/10.1093/nar/gkr466
Meesapyodsuk D, Qiu X (2016) Biosynthetic mechanism of very long chain polyunsaturated fatty acids in Thraustochytrium sp. 26185. J Lipid Res Submitted 57(10):1–39. https://doi.org/10.1194/jlr.M070136
Meesapyodsuk D, Qiu X (2012) The front-end desaturase: structure, function, evolution and biotechnological use. Lipids 47(3):227–237. https://doi.org/10.1007/s11745-011-3617-2
Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J (2001) Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293(80):290–292. https://doi.org/10.1126/science.1059593
Okuyama H, Orikasa Y, Nishida T, Watanabe K, Morita N (2007) Bacterial genes responsible for the biosynthesis of eicosapentaenoic and docosahexaenoic acids and their heterologous expression. Appl Environ Microbiol 73(3):665–670. https://doi.org/10.1128/AEM.02270-06
Parsons JB, Rock CO (2013) Progress in lipid research bacterial lipids : metabolism and membrane homeostasis. Prog Lipid Res 52(3):249–276. https://doi.org/10.1016/j.plipres.2013.02.002
Pasta S, Witkowski A, Joshi AK, Smith S (2007) Catalytic residues are shared between two pseudosubunits of the dehydratase domain of the animal fatty acid synthase. Chem Biol 14(12):1377–1385. https://doi.org/10.1016/j.chembiol.2007.11.007
Qiu X (2003) Biosynthesis of docosahexaenoic acid (DHA, 22:6-4, 7,10,13,16,19): two distinct pathways. Prostaglandins Leukot Essent Fat Acids 68(2):181–186. https://doi.org/10.1016/S0952-3278(02)00268-5
Rawsthorne S (2002) Carbon flux and fatty acid synthesis in plants. 41:182–196. doi:https://doi.org/10.1016/S0163-7827(01)00023-6
Rock CO (2008) Fatty acid and phospholipid metabolism in prokaryotes. Biochem Lipids, Lipoproteins Membr:59–96. https://doi.org/10.1016/B978-044453219-0.50005-2
Rock CO, Tsay JT, Heath R, Jackowski S (1996) Increased unsaturated fatty acid production associated with a suppressor of the fabA6(Ts) mutation in Escherichia coli. J Bacteriol 178(18):5382–5387. https://doi.org/10.1128/jb.178.18.5382-5387.1996
Rouhiainen L, Vakkilainen T, Siemer BL, Buikema W, Haselkorn R, Sivonen K (2004) Genes coding for hepatotoxic heptapeptides (microcystins) in the Cyanobacterium Anabaena strain 90. Appl Environ Microbiol 70(2):686–692. https://doi.org/10.1128/AEM.70.2.686-692.2004
Sheppard D (1994) Dominant negative mutants: tools for the study of protein function in vitro and in vivo. Am J Respir Cell Mol Biol 11(1):1–6. https://doi.org/10.1165/ajrcmb.11.1.8018332
Smith S, Witkowski A, Joshi AK (2003) Structural and functional organization of the animal fatty acid synthase. Prog Lipid Res 42(4):289–317. https://doi.org/10.1016/S0163-7827(02)00067-X
Yamazaki Y, Sugawara H (2009) National BioResource Project Information Center. Exp Anim Japanese Assoc Lab Anim Sci 58(2):75–84. https://doi.org/10.1538/expanim.58.75
Xie X, Meesapyodsuk D, Qiu X (2017) Ketoacylsynthase domains of a PUFA synthase in Thraustochytrium can effectively function as stand-alone enzymes in Escherichia coli. Appl Env Microbiol 83(9):e03133–e03116. https://doi.org/10.1128/AEM.03133-16
Zhang S, Sakuradani E, Ito K, Shimizu S (2007) Identification of a novel bifunctional Δ12/Δ15 fatty acid desaturase from a basidiomycete, Coprinus cinereus TD#822-2. FEBS Lett 581(2):315–319. https://doi.org/10.1016/j.febslet.2006.12.031
Zhao X, Meesapyodsuk D, Qu C, Qiu X (2016) Genomic analysis of genes involved in the biosynthesis of very long chain polyunsaturated fatty acids in Thraustochytrium sp. 26185. Lipids 51(9):1065–1075. https://doi.org/10.1007/s11745-016-4181-6
Acknowledgments
We thank Dr. Charles Rock, Department of Biochemistry, St. Jude Children’s Research Hospital, Tennessee, USA for providing the plasmid with E. coli FabA gene and National BioResource Project (NBRP E. coli, Microbial Genetics Laboratory, National Institute of Genetics, Japan) for providing E. coli mutant strains.
Funding
This research was supported by Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 215 kb)
Rights and permissions
About this article
Cite this article
Xie, X., Meesapyodsuk, D. & Qiu, X. Functional analysis of the dehydratase domains of a PUFA synthase from Thraustochytrium in Escherichia coli . Appl Microbiol Biotechnol 102, 847–856 (2018). https://doi.org/10.1007/s00253-017-8635-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8635-4