Abstract
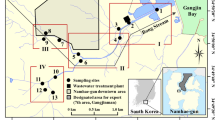
The fecal bacteria in natural waters may pose serious risks on human health. Although many source tracking methods have been developed and used to determine the possible sources of the fecal pollution, little is known about the overall diversity and abundance of fecal bacterial community in natural waters. In this study, a method based on fecal bacterial sequence library was introduced to evaluate the fecal bacterial profile in the Yangtze River (Nanjing section). Our results suggested that the Yangtze River water harbors diverse fecal bacteria. Fifty-eight fecal operational taxonomic units (97% identity level) were detected in the Yangtze River water samples and the relative abundance of fecal bacteria in these samples ranged from 0.1 to 8%. It was also found that the relative abundances of the fecal bacteria in locations near to the downstream of wastewater treatment plants were obviously higher than those in other locations. However, the high abundance of fecal bacteria could decrease to the normal level in 2~4 km in the river due to degradation or dilution, and the overall fecal bacteria level changed little when the Yangtze River flew through the Nanjing City. Moreover, the fecal bacteria in the Yangtze River water were found to be highly associated (Spearman rho = 0.804, P < 0.001) with the potential pathogenic bacteria. Collectively, the findings in this study reveal the diversity, abundance, and possible sources of fecal bacteria in the Yangtze River and advance our understandings of the fecal bacteria community in the natural waters.






Similar content being viewed by others
References
Ahmed W, Staley C, Sadowsky M, Gyawali P, Sidhu J, Palmer A, Beale D, Toze S (2015) Toolbox approaches using molecular markers and 16S rRNA gene amplicon data sets for identification of fecal pollution in surface water. Appl Environ Microbiol 81(20):7067–7077
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Ballesté E, Blanch AR (2010) Persistence of Bacteroides species populations in a river as measured by molecular and culture techniques. Appl Environ Microbiol 76(22):7608–7616
Bibby K, Viau E, Peccia J (2010) Pyrosequencing of the 16S rRNA gene to reveal bacterial pathogen diversity in biosolids. Water Res 44(14):4252–4260
Boehm AB, Van De Werfhorst LC, Griffith JF, Holden PA, Jay JA, Shanks OC, Wang D, Weisberg SB (2013) Performance of forty-one microbial source tracking methods: a twenty-seven lab evaluation study. Water Res 47(18):6812–6828
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Chen H, Shu W, Chang X, J-a C, Guo Y, Tan Y (2010) The profile of antibiotics resistance and integrons of extended-spectrum beta-lactamase producing thermotolerant coliforms isolated from the Yangtze River basin in Chongqing. Environ Pollut 158(7):2459–2464
Chen J, Chen Z, Xu K, Wei T, Li M, Wang Z, Watanabe M (2005) ADP-flow velocity profile to interpret hydromorphological features of China’s Yangtze Three-Gorges valley. Chin Sci Bull 50(7):679–684
Chihomvu P, Stegmann P, Pillay M (2015) Characterization and structure prediction of partial length protein sequences of pcoA, pcoR and chrB genes from heavy metal resistant bacteria from the Klip River, South Africa. Int J Mol Sci 16(4):7352–7374
Costa MC, Arroyo LG, Allen-Vercoe E, Stämpfli HR, Kim PT, Sturgeon A, Weese JS (2012) Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS One 7(7):e41484
Crane SR, Moore JA (1986) A management strategy to reduce bacterial pollution in shellfish areas: a case study. Environ Manag 10(1):41–51
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461
George I, Crop P, Servais P (2002) Fecal coliform removal in wastewater treatment plants studied by plate counts and enzymatic methods. Water Res 36(10):2607–2617
Hong PY, Wu JH, Liu WT (2009) A high-throughput and quantitative hierarchical oligonucleotide primer extension (HOPE)-based approach to identify sources of faecal contamination in water bodies. Environ Microbiol 11(7):1672–1681
Knights D, Kuczynski J, Charlson ES, Zaneveld J, Mozer MC, Collman RG, Bushman FD, Knight R, Kelley ST (2011) Bayesian community-wide culture-independent microbial source tracking. Nat Methods 8(9):761–763
Lee JE, Lee S, Sung J, Ko G (2011) Analysis of human and animal fecal microbiota for microbial source tracking. The ISME journal 5(2):362–365
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444(7122):1022–1023
Lipp EK, Farrah SA, Rose JB (2001) Assessment and impact of microbial fecal pollution and human enteric pathogens in a coastal community. Mar Pollut Bull 42(4):286–293
Liu X, Fan H, Ding X, Hong Z, Nei Y, Liu Z, Li G, Guo H (2014) Analysis of the gut microbiota by high-throughput sequencing of the V5–V6 regions of the 16S rRNA gene in donkey. Curr Microbiol 68(5):657–662
Mao S, Zhang R, Wang D, Zhu W (2012) The diversity of the fecal bacterial community and its relationship with the concentration of volatile fatty acids in the feces during subacute rumen acidosis in dairy cows. BMC Vet Res 8(1):1
Marti R, Gannon VP, Jokinen C, Lanthier M, Lapen DR, Neumann NF, Ruecker NJ, Scott A, Wilkes G, Zhang Y (2013) Quantitative multi-year elucidation of fecal sources of waterborne pathogen contamination in the South Nation River basin using Bacteroidales microbial source tracking markers. Water Res 47(7):2315–2324
Parks DH, Tyson GW, Hugenholtz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30(21):3123–3124
Saito Y, Yang Z, Hori K (2001) The Huanghe (Yellow River) and Changjiang (Yangtze River) deltas: a review on their characteristics, evolution and sediment discharge during the Holocene. Geomorphology 41(2):219–231
Savio D, Sinclair L, Ijaz UZ, Parajka J, Reischer GH, Stadler P, Blaschke AP, Blöschl G, Mach RL, Kirschner AK (2015) Bacterial diversity along a 2600 km river continuum. Environ Microbiol 17(12):4994–5007
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75(23):7537–7541
Servais P, Garcia-Armisen T, George I, Billen G (2007) Fecal bacteria in the rivers of the Seine drainage network (France): sources, fate and modelling. Sci Total Environ 375(1):152–167
Staley C, Unno T, Gould T, Jarvis B, Phillips J, Cotner J, Sadowsky M (2013) Application of Illumina next-generation sequencing to characterize the bacterial community of the Upper Mississippi River. J Appl Microbiol 115(5):1147–1158
Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66(11):5066–5072
Unno T, Jang J, Han D, Kim JH, Sadowsky MJ, Kim O-S, Chun J, Hur H-G (2010) Use of barcoded pyrosequencing and shared OTUs to determine sources of fecal bacteria in watersheds. Environ Sci Technol 44(20):7777–7782
Vilanova X, Manero A, Cerdà-Cuéllar M, Blanch A (2002) The effect of a sewage treatment plant effluent on the faecal coliforms and enterococci populations of the reception river waters. J Appl Microbiol 92(2):210–214
Wéry N, Monteil C, Pourcher A-M, Godon J-J (2010) Human-specific fecal bacteria in wastewater treatment plant effluents. Water Res 44(6):1873–1883
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267
Ye L, Zhang T (2011) Pathogenic bacteria in sewage treatment plants as revealed by 454 pyrosequencing. Environ Sci Technol 45(17):7173–7179
Ye XY, Ming X, Zhang YL, Xiao WQ, Huang XN, Cao YG, Gu KD (2012) Real-time PCR detection of enteric viruses in source water and treated drinking water in Wuhan, China. Curr Microbiol 65(3):244–253
Acknowledgements
The authors thank Dr. Peng Shi for assisting in water sampling.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This study was funded by the China Fundamental Research Funds for the Central Universities.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 608 kb)
Rights and permissions
About this article
Cite this article
Sun, H., He, X., Ye, L. et al. Diversity, abundance, and possible sources of fecal bacteria in the Yangtze River. Appl Microbiol Biotechnol 101, 2143–2152 (2017). https://doi.org/10.1007/s00253-016-7998-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7998-2