Abstract
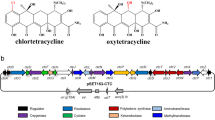
Heterologous expression is an important strategy to activate biosynthetic gene clusters of secondary metabolites. Here, it is employed to activate and manipulate the oxytetracycline (OTC) gene cluster and to alter OTC fermentation process. To achieve these goals, a fast-growing heterologous host Streptomyces venezuelae WVR2006 was rationally selected among several potential hosts. It shows rapid and dispersed growth and intrinsic high resistance to OTC. By manipulating the expression of two cluster-situated regulators (CSR) OtcR and OtrR and precursor supply, the OTC production level was significantly increased in this heterologous host from 75 to 431 mg/l only in 48 h, a level comparable to the native producer Streptomyces rimosus M4018 in 8 days. This work shows that S. venezuelae WVR2006 is a promising chassis for the production of secondary metabolites, and the engineered heterologous OTC producer has the potential to completely alter the fermentation process of OTC production.





Similar content being viewed by others
References
Agwuh KN, MacGowan A (2006) Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265. doi:10.1093/jac/dkl224
Bilyk O, Luzhetskyy A (2016) Metabolic engineering of natural product biosynthesis in actinobacteria. Curr Opin Biotechnol 42:98–107. doi:10.1016/j.copbio.2016.03.008
Chu X, Zhen Z, Tang Z (2012) Introduction of extra copy of oxytetracycline resistance Gene otrB enhances the biosynthesis of oxytetracycline in Streptomyces rimosus. J Bioproces Biotechniq 02:1–4. doi:10.4172/2155-9821.1000117
Elizarov SM, Alekseeva MG, Novikov FN, Chilov GG, Maslov DA, Shtil AA, Danilenko VN (2012) Identification of phosphorylation sites in aminoglycoside phosphotransferase VIII from Streptomyces rimosus. Biochemistry (Mosc) 77:1258–1265. doi:10.1134/S0006297912110041
Fan K, Pan G, Peng X, Zheng J, Gao W, Wang J, Wang W, Li Y, Yang K (2012) Identification of JadG as the B ring opening oxygenase catalyzing the oxidative C-C bond cleavage reaction in jadomycin biosynthesis. Chem Biol 19:1381–1390. doi:10.1016/j.chembiol.2012.09.009
Galm U, Shen B (2006) Expression of biosynthetic gene clusters in heterologous hosts for natural product production and combinatorial biosynthesis. Expert Opin Drug Discov 1:409–437. doi:10.1517/17460441.1.5.409
Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA 3rd, Smith HO (2009) Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi:10.1038/nmeth.1318
Gomez-Escribano JP, Bibb MJ (2011) Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Methods Enzymol 4:207–215. doi:10.1111/j.1751-7915.2010.00219.x
Gust B, Challis GL, Fowler K, Kieser T, Chater KF (2003) PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc Natl Acad Sci U S A 100:1541–1546. doi:10.1073/pnas.0337542100
Jiang W, Zhao X, Gabrieli T, Lou C, Ebenstein Y, Zhu TF (2015) Cas9-assisted targeting of CHromosome segments CATCH enables one-step targeted cloning of large gene clusters. Nat Commun 6:8101. doi:10.1038/ncomms9101
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics. The John Innes Foundation, Norwich
Kim EJ, Lee JH, Choi H, Pereira AR, Ban YH, Yoo YJ, Kim E, Park JW, Sherman DH, Gerwick WH, Yoon YJ (2012) Heterologous production of 4-O-demethylbarbamide, a marine cyanobacterial natural product. Org Lett 14:5824–5827. doi:10.1021/ol302575h
Kim EJ, Yang I, Yoon YJ (2015) Developing Streptomyces venezuelae as a cell factory for the production of small molecules used in drug discovery. Arch Pharm Res 38:1606–1616. doi:10.1007/s12272-015-0638-z
Labes G, Bibb M, Wohlleben W (1997) Isolation and characterization of a strong promoter element from the Streptomyces ghanaensis phage I19 using the gentamicin resistance gene (aacC1) of Tn 1696 as reporter. Microbiology 143(Pt 5):1503–1512
Lesnik UaG A, Magdevska V, Fujs S, Raspor P, Hunter I, Petkovic H (2009) Regulatory elements in tetracycline-encoding gene clusters-the otcG gene positively regulates the production of oxytetracycline in Streptomyces rimosus. Food Technol Biotechnol 47:323–330
Li S, Wang W, Li X, Fan K, Yang K (2015) Genome-wide identification and characterization of reference genes with different transcript abundances for Streptomyces coelicolor. Sci Rep 5:15840. doi:10.1038/srep15840
Luo Y, Enghiad B, Zhao H (2016) New tools for reconstruction and heterologous expression of natural product biosynthetic gene clusters. Nat Prod Rep 33:174–182. doi:10.1039/c5np00085h
Nelson ML, Levy SB (2011) The history of the tetracyclines. Ann N Y Acad Sci 1241:17–32. doi:10.1111/j.1749-6632.2011.06354.x
Onaka H, Ozaki T, Mori Y, Izawa M, Hayashi S, Asamizu S (2015) Mycolic acid-containing bacteria activate heterologous secondary metabolite expression in Streptomyces lividans. J Antibiot (Tokyo) 68:594–597. doi:10.1038/ja.2015.31
Ongley SE, Bian X, Neilan BA, Muller R (2013) Recent advances in the heterologous expression of microbial natural product biosynthetic pathways. Nat Prod Rep 30:1121–1138. doi:10.1039/c3np70034h
Park SR, Ahn MS, Han AR, Park JW, Yoon YJ (2011) Enhanced flavonoid production in Streptomyces venezuelae via metabolic engineering. J Microbiol Biotechnol 21:1143–1146
Peric-Concha N, Borovicka B, Long PF, Hranueli D, Waterman PG, Hunter IS (2005) Ablation of the otcC gene encoding a post-polyketide hydroxylase from the oxytetracyline biosynthetic pathway in Streptomyces rimosus results in novel polyketides with altered chain length. J Biol Chem 280:37455–37460. doi:10.1074/jbc.M503191200
Petkovic H, Thamchaipenet A, Zhou LH, Hranueli D, Raspor P, Waterman PG, Hunter IS (1999) Disruption of an aromatase/cyclase from the oxytetracycline gene cluster of Streptomyces rimosus results in production of novel polyketides with shorter chain lengths. J Biol Chem 274:32829–32834
Pickens LB, Tang Y (2010) Oxytetracycline biosynthesis. J Biol Chem 285:27509–27515. doi:10.1074/jbc.R110.130419
Pullan ST, Chandra G, Bibb MJ, Merrick M (2011) Genome-wide analysis of the role of GlnR in Streptomyces venezuelae provides new insights into global nitrogen regulation in actinomycetes. BMC Genomics 12:175. doi:10.1186/1471-2164-12-175
Sekurova ON, Brautaset T, Sletta H, Borgos SE, Jakobsen MO, Ellingsen TE, Strom AR, Valla S, Zotchev SB (2004) In vivo analysis of the regulatory genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455 reveals their differential control over antibiotic biosynthesis. J Bacteriol 186:1345–1354
Stuttard C (1983) Localized hydroxylamine mutagenesis, and cotransduction of threonine and lysine genes, in Streptomyces venezuelae. J Bacteriol 155:1219–1223
Tang Z, Xiao C, Zhuang Y, Chu J, Zhang S, Herron PR, Hunter IS, Guo M (2011) Improved oxytetracycline production in Streptomyces rimosus M4018 by metabolic engineering of the G6PDH gene in the pentose phosphate pathway. Enzym Microb Technol 49:17–24. doi:10.1016/j.enzmictec.2011.04.002
Wang B, Ren J, Li L, Guo F, Pan G, Ai G, Aigle B, Fan K, Yang K (2015) Kinamycin biosynthesis employs a conserved pair of oxidases for B-ring contraction. Chem Commun (Camb) 51:8845–8848. doi:10.1039/c5cc01986a
Wang L, Tian X, Wang J, Yang H, Fan K, Xu G, Yang K, Tan H (2009a) Autoregulation of antibiotic biosynthesis by binding of the end product to an atypical response regulator. Proc Natl Acad Sci U S A 106:8617–8622. doi:10.1073/pnas.0900592106
Wang P, Zhang W, Zhan J, Tang Y (2009b) Identification of OxyE as an ancillary oxygenase during tetracycline biosynthesis. Chembiochem 10:1544–1550. doi:10.1002/cbic.200900122
Wang P, Gao X, Chooi YH, Deng Z, Tang Y (2011) Genetic characterization of enzymes involved in the priming steps of oxytetracycline biosynthesis in Streptomyces rimosus. Microbiology 157:2401–2409. doi:10.1099/mic.0.048439-0
Wang P, Kim W, Pickens LB, Gao X, Tang Y (2012) Heterologous expression and manipulation of three tetracycline biosynthetic pathways. Angew Chem Int Ed 51(44):11136–11140. doi:10.1002/anie.201205426
Wang P, Bashiri G, Gao X, Sawaya MR, Tang Y (2013a) Uncovering the enzymes that catalyze the final steps in oxytetracycline biosynthesis. J Am Chem Soc 135:7138–7141. doi:10.1021/ja403516u
Wang W, Li X, Wang J, Xiang S, Feng X, Yang K (2013b) An engineered strong promoter for streptomycetes. Appl Environ Microbiol 79:4484–4492. doi:10.1128/AEM.00985-13
Wang W, Yang T, Li Y, Li S, Yin S, Styles K, Corre C, Yang K (2016) Development of a synthetic oxytetracycline-inducible expression system for Streptomycetes using de novo characterized genetic parts. ACS Synth Biol. doi:10.1021/acssynbio.6b00087
Xu G, Wang J, Wang L, Tian X, Yang H, Fan K, Yang K, Tan H (2010) “Pseudo” gamma-butyrolactone receptors respond to antibiotic signals to coordinate antibiotic biosynthesis. J Biol Chem 285:27440–27448. doi:10.1074/jbc.M110.143081
Yang K, Han L, He J, Wang L, Vining LC (2001) A repressor-response regulator gene pair controlling jadomycin B production in Streptomyces venezuelae ISP5230. Gene 279:165–173
Yang K, Han L, Vining LC (1995) Regulation of jadomycin B production in Streptomyces venezuelae ISP5230: involvement of a repressor gene, jadR2. J Bacteriol 177:6111–6117
Yin S, Wang W, Wang X, Zhu Y, Jia X, Li S, Yuan F, Zhang Y, Yang K (2015) Identification of a cluster-situated activator of oxytetracycline biosynthesis and manipulation of its expression for improved oxytetracycline production in Streptomyces rimosus. Microb Cell Factories 14:1–12. doi:10.1186/s12934-015-0231-7
Yu L, Cao N, Wang L, Xiao C, Guo M, Chu J, Zhuang Y, Zhang S (2012) Oxytetracycline biosynthesis improvement in Streptomyces rimosus following duplication of minimal PKS genes. Enzym Microb Technol 50:318–324. doi:10.1016/j.enzmictec.2012.03.001
Zaburannyi N, Rabyk M, Ostash B, Fedorenko V, Luzhetskyy A (2014) Insights into naturally minimised Streptomyces albus J1074 genome. BMC Genomics 15:97. doi:10.1186/1471-2164-15-97
Zhang W, Ames BD, Tsai SC, Tang Y (2006) Engineered biosynthesis of a novel amidated polyketide, using the malonamyl-specific initiation module from the oxytetracycline polyketide synthase. Appl Environ Microbiol 72:2573–2580. doi:10.1128/AEM.72.4.2573-2580.2006
Zhang W, Watanabe K, Wang CC, Tang Y (2007) Investigation of early tailoring reactions in the oxytetracycline biosynthetic pathway. J Biol Chem 282:25717–25725. doi:10.1074/jbc.M703437200
Zhang Y, Muyrers JP, Testa G, Stewart AF (2000) DNA cloning by homologous recombination in Escherichia coli. Nat Biotechnol 18:1314–1317. doi:10.1038/82449
Zhang Y, Pan G, Zou Z, Fan K, Yang K, Tan H (2013) JadR*-mediated feed-forward regulation of cofactor supply in jadomycin biosynthesis. Mol Microbiol 90:884–897. doi:10.1111/mmi.12406
Acknowledgments
The authors wish to thank Prof. Meijin Guo (East China University of Science and Technology) for providing S. rimosus M4018. This work was supported by funding from Shengxue Dacheng Pharmaceutical Co., Ltd., the National Natural Science Foundation of China (Grant Nos. 31400034 and 31570031) and the Ministry of Science and Technology of China (Grant No. 2013CB734001). Weishan Wang is an awardee of Youth Innovation Promotion Association of CAS [92016087]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Shouliang Yin and Zilong Li contributed equally to this work.
Electronic supplementary material
ESM 1
(PDF 271 kb)
Rights and permissions
About this article
Cite this article
Yin, S., Li, Z., Wang, X. et al. Heterologous expression of oxytetracycline biosynthetic gene cluster in Streptomyces venezuelae WVR2006 to improve production level and to alter fermentation process. Appl Microbiol Biotechnol 100, 10563–10572 (2016). https://doi.org/10.1007/s00253-016-7873-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7873-1