Abstract
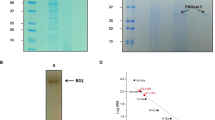
β-Glucosidase (BG) is widely applied in the biofuel’s industry, as part of a cellulase cocktail to catalyze the hydrolysis of the β-1,4 linkages that join two glucose molecules in a cellulose polymer. The hydrolysis step is generally recognized as the major limiting step in the development of efficient enzyme-based technologies for the conversion of lignocellulosic biomass to sugars and the production of biofuels due to the accumulation of the reaction product, glucose. Relieving this glucose inhibition of BG is therefore a major challenge. In this study, O08324, a putative BG gene encoded in the hyperthermophilic archaeon Thermococcus sp., was cloned and overexpressed in Escherichia coli. O08324 showed maximum activity between pH 5–6.8 and at 78 °C and was thermostable with a half-life of 860 min at 78 °C in the presence of 1.5 M glucose. O08324 was not inhibited by glucose up to the highest assayable concentration of 4 M and also shows no decrease in activity in the presence of up to 4 M of sodium chloride or potassium chloride. O08324 supplementation of Trichoderma viride cellulase enhanced glucose production by more than 50 % compared to a commercially available BG, when Avicel (10 %, w/v) was used as a substrate at 37 °C. Multiple sequence alignments across previously reported glucose-tolerant BGs shows that many conserved residues previously implicated in glucose tolerance are not conserved in this BG suggesting a need for a relook at understanding the molecular basis of glucose tolerance.






Similar content being viewed by others
References
Bauer MW, Driskill LE, Kelly RM (1998) Glycosyl hydrolases from hyperthermophilic microorganisms. Curr Opin Biotechnol 9(2):141–145
Cao L-C, Wang Z-J, Ren G-H, Kong W, Li L, Xie W, Liu Y-H (2015) Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol Biofuel 8(1):1–12
Datta S (2016) Recent strategies to overexpress and engineer cellulases for biomass degradation. Curr Metabolomics 4(1):14–22
de Giuseppe PO, Souza Tde A, Souza FH, Zanphorlin LM, Machado CB, Ward RJ, Jorge JA, Furriel Rdos P, Murakami MT (2014) Structural basis for glucose tolerance in GH1 β-glucosidases. Acta Crystallogr D Biol Crystallogr D70:1631–1639
Egorova K, Antranikian G (2005) Industrial relevance of thermophilic archaea. Curr Opin Microbiol 8(6):649–655
Fang Z, Fang W, Liu J, Hong Y, Peng H, Zhang X (2010) Cloning and characterization of a β-glucosidase from marine microbial metagenome with excellent glucose tolerance. J Microbiol Biotechnol 20(9):1351–1358
Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (ed) The proteomics protocols handbook. Humana Press, Totowa, pp. 571–607
Goswami S, Gupta N, Datta S (2016) Using the β-glucosidase catalyzed reaction product glucose to improve the ionic liquid tolerance of β-glucosidases. Biotechnol Biofuels 9(1):1–12
Graham JE, Clark ME, Nadler DC, Huffer S, Chokhawala HA, Rowland SE, Blanch HW, Clark DS, Robb FT (2011) Identification and characterization of a multidomain hyperthermophilic cellulase from an archaeal enrichment. Nat Commun 2:375
Gunny AAN, Arbain D, Edwin Gumba R, Jong BC, Jamal P (2014) Potential halophilic cellulases for in situ enzymatic saccharification of ionic liquids pretreated lignocelluloses. Bioresour Technol 155:177–181
Hassan N, Nguyen TH, Intanon M, Kori LD, Patel BK, Haltrich D, Divne C, Tan TC (2015) Biochemical and structural characterization of a thermostable β-glucosidase from Halothermothrix orenii for galacto-oligosaccharide synthesis. Appl Microbiol Biotechnol 99(4):1731–1744
Heins RA, Cheng X, Nath S, Deng K, Bowen BP, Chivian DC, Datta S, Friedland GD, D’Haeseleer P, Wu D, Tran-Gyamfi M, Scullin CS, Singh S, Shi W, Hamilton MG, Bendall ML, Sczyrba A, Thompson J, Feldman T, Guenther JM, Gladden JM, Cheng J-F, Adams PD, Rubin EM, Simmons BA, Sale KL, Northen TR, Deutsch S (2014) Phylogenomically guided identification of industrially relevant GH1 β-glucosidases through DNA synthesis and nanostructure-initiator mass spectrometry. ACS Chem Biol 9(9):2082–2091
Kaushik JK, Bhat R (1999) A mechanistic analysis of the increase in the thermal stability of proteins in aqueous carboxylic acid salt solutions. Protein Sci 8(1):222–233
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzym Res 2011:280696
Kushner D, Kamekura M (1988) Physiology of halophilic eubacteria. In: Rodríguez-Varela F (ed) Halophilic bacteria, vol 1. CRC Press, Boca Raton, pp. 109–138
Lee HL, Chang CK, Jeng WY, Wang AH, Liang PH (2012) Mutations in the substrate entrance region of β-glucosidase from Trichoderma reesei improve enzyme activity and thermostability. Protein Eng Des Sel 25(11):733–740
Leis B, Heinze S, Angelov A, Pham VT, Thurmer A, Jebbar M, Golyshin PN, Streit WR, Daniel R, Liebl W (2015) Functional screening of hydrolytic activities reveals an extremely thermostable cellulase from a deep-sea archaeon. Front Bioeng Biotechnol 3:95
Li G, Jiang Y, Fan XJ, Liu YH (2012) Molecular cloning and characterization of a novel β-glucosidase with high hydrolyzing ability for soybean isoflavone glycosides and glucose-tolerance from soil metagenomic library. Bioresour Technol 123:15–22
Liszka MJ, Clark ME, Schneider E, Clark DS (2012) Nature versus nurture: developing enzymes that function under extreme conditions. Annu Rev Chem Biomol Eng 3:77–102
Lu J, Du L, Wei Y, Hu Y, Huang R (2013) Expression and characterization of a novel highly glucose-tolerant β-glucosidase from a soil metagenome. Acta Biochim Biophys Sin Shanghai 45(8):664–673
Mai Z, Yang J, Tian X, Li J, Zhang S (2013) Gene cloning and characterization of a novel salt-tolerant and glucose-enhanced β-glucosidase from a marine streptomycete. Appl Biochem Biotechnol 169(5):1512–1522
Matsui I, Sakai Y, Matsui E, Kikuchi H, Kawarabayasi Y, Honda K (2000) Novel substrate specificity of a membrane-bound β-glycosidase from the hyperthermophilic archaeon Pyrococcus horikoshii. FEBS Lett 467(2–3):195–200
Niesen FH, Berglund H, Vedadi M (2007) The use of differential scanning fluorimetry to detect ligand interactions that promote protein stability. Nat Protoc 2(9):2212–2221
Riou C, Salmon JM, Vallier MJ, Gunata Z, Barre P (1998) Purification, characterization, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl Environ Microbiol 64(10):3607–3614
Saha B, Bothast R (1996) Production, purification and characterization of a highly glucose-tolerant novel β-glucosidase from Candida peltata. Appl Environ Microbiol 62(9):3165–3170
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539
Sternberg D, Vijayakumar P, Reese ET (1977) β-Glucosidase: microbial production and effect on enzymatic hydrolysis of cellulose. Can J Microbiol 23(2):139–147
Teugjas H, Väljamäe P (2013) Selecting β-glucosidases to support cellulases in cellulose saccharification. Biotechnol Biofuels 6(1):105
Uchiyama T, Miyazaki K, Yaoi K (2013) Characterization of a novel β-glucosidase from a compost microbial metagenome with strong transglycosylation activity. J Biol Chem 288(25):18325–18334
Yan TR, Lin CL (1997) Purification and characterization of a glucose-tolerant β-glucosidase from Aspergillus niger CCRC 31494. Biosci Biotechnol Biochem 61(6):965–970
Yang Y, Zhang X, Yin Q, Fang W, Fang Z, Wang X, Xiao Y (2015) A mechanism of glucose tolerance and stimulation of GH1 β-glucosidases. Sci Rep 5:17296
Yeoman CJ, Han Y, Dodd D, Schroeder CM, Mackie RI, Cann IK (2010) Thermostable enzymes as biocatalysts in the biofuel industry. Adv Appl Microbiol 70:1–55
Zhang T, Datta S, Eichler J, Ivanova N, Axen SD, Kerfeld CA, Chen F, Kyrpides N, Hugenholtz P, Cheng J-F, Sale KL, Simmons B, Rubin E (2011) Identification of a haloalkaliphilic and thermostable cellulase with improved ionic liquid tolerance. Green Chem 13(8):2083–2090
Acknowledgments
This work was supported in part by Rapid Grant for Young Investigators, Department of Biotechnology, Government of India, BT/PR6511/GBD/27/424/2012 (S.D.); Energy Bioscience Overseas Fellowship, Department of Biotechnology, Government of India, BT/NBDB/22/06/2011 (S.D); and IISER Kolkata Academic Research Fund. S.K.S is supported by a Junior Research Fellowship from CSIR, Govt. of India. We thank Ms. Baishali Roy for her help in the cloning and expression studies.
Authors’ contributions
S.D. and S.K.S designed the research. S.K.S performed the experiments. S.D. wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 3944 kb)
Rights and permissions
About this article
Cite this article
Sinha, S.K., Datta, S. β-Glucosidase from the hyperthermophilic archaeon Thermococcus sp. is a salt-tolerant enzyme that is stabilized by its reaction product glucose. Appl Microbiol Biotechnol 100, 8399–8409 (2016). https://doi.org/10.1007/s00253-016-7601-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7601-x