Abstract
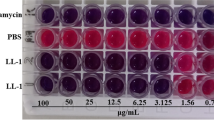
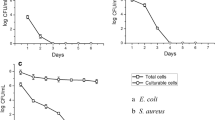
This work is the first to report the antibacterial characteristics and antibacterial mechanisms of MP1102, which is a variant of NZ2114, against pathogenic Clostridium perfringens. MP1102 exhibited strong antimicrobial activity against C. perfringens strains CVCC 61, CVCC 1163, and CVCC 2032 at a low minimal inhibitory concentration (MIC) of 0.91 μM. MP1102 showed anti-C. perfringens activity over a wide pH range of 2.0 and 10.0, high thermal stability from 20 to 80 °C, and remarkable resistance to pepsin. The fractional inhibitory concentration index (FICI) indicated an additive or synergic effect between MP1102 and bacitracin zinc, nisin, vancomycin, virginiamycin, aureomycin, and ampicillin against C. perfringens (FICI = 0.3125–1.0). To further elucidate the antibacterial mechanism of MP1102, its effect on the C. perfringens CVCC 61 cell membrane and intracellular DNA was studied. Flow cytometry and scanning electron microscopy (SEM) indicated that MP1102 treatment resulted in the release of cellular contents by damaging the membrane. A DNA gel retardation and circular dichroism analysis demonstrated that MP1102 interacted with DNA and intercalated into the DNA base pairs. A cell cycle assay demonstrated that MP1102 affected cellular functions, such as DNA synthesis. These results suggested that MP1102 exhibited potential as a new antimicrobial agent against C. perfringens infections.






Similar content being viewed by others
References
Adams V, Watts TD, Bulach DM, Lyras D, Rood JI (2015) Plasmid partitioning systems of conjugative plasmids from Clostridium perfringens. Plasmid 80(2015):90–96
Anunthawan T, de la Fuente-Núñez C, Hancock RE, Klaynongsruang S (2015) Cationic amphipathic peptides KT2 and RT2 are taken up into bacterial cells and kill planktonic and biofilmbacteria. Biochim Biophys Acta 848(6):1352–1358
Brinch KS, Sandberg A, Baudoux P, Van Bambeke F, Tulkens PM, Frimodt-Møller N, Høiby N, Kristensen HH. Plectasin shows intracellular activity against Staphylococcus aureus in human THP-1 monocytes and in a mouse peritonitis model (2009) Antimicrob Agents Chemother 53(11): 4801–4808
Cervantes HM (2015) Antibiotic-free poultry production: is it sustainable? J Appl Poult Res 24(1):91–97
Colen CB, Rayes M, Rengachary S, Guthikonda M (2007) Outcome of brain abscess by Clostridium perfringens. Neurosurgery 61(6):E1339
Cao X, Zhang Y, Mao R, Teng D, Wang X, Wang J (2015) Design and recombination expression of a novel plectasin-derived peptide MP1106 and its properties against Staphylococcus aureus. Appl Microbiol Biotechnol 99(6):2649–2662
Dong N, Zhu X, Chou S, Shan A, Li W, Jiang J (2014) Antimicrobial potency and selectivity of simplified symmetric-end peptides. Biomaterials 35(27):8028–8039
Eckert R (2011) Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol 6(6):635–651
Gaucher ML, Quessy S, Letellier A, Arsenault J, Boulianne M (2015) Impact of a drug-free program on broiler chicken growth performances, gut health, Clostridium perfringens and Campylobacter jejuni occurrences at the farm level. Poult Sci 94(8):1791–1801
Gholamiandehkordi A, Eeckhaut V, Lanckriet A, Timbermont L, Bjerrum L, Ducatelle R, Haesebrouck F, Van Immerseel F (2009) Antimicrobial resistance in Clostridium perfringens isolates from broilers in Belgium. Vet Res Commun 33(8):1031–1037
Giguère S, Lee EA, Guldbech KM, Berghaus LJ (2012) In vitro synergy, pharmacodynamics, and postantibiotic effect of 11 antimicrobial agents against Rhodococcus equi. Vet Microbiol 160(1–2):207–213
Grass JE, Gould LH, Mahon BE (2013) Epidemiology of foodborne disease outbreaks caused by Clostridium perfringens, United States, 1998-2010. Foodborne Pathog Dis 10(2):131–136
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557
Hara S, Mukae H, Sakamoto N, Ishimoto H, Amenomori M, Fujita H, Ishimatsu Y, Yanagihara K, Kohno S (2008) Plectasin has antibacterial activity and no affect on cell viability or IL-8 production. Biochem Biophys Res Commun 374(4):709–713
Hölzel CS, Harms KS, Schwaiger K, Bauer J (2010) Resistance to linezolid in a porcine Clostridium perfringens strain carrying a mutation in the rplD Gene encoding the ribosomal protein L4. Antimicrob Agents Chemother 54(3):1351–1353
Li J, Adams V, Bannam TL, Miyamoto K, Garcia JP, Uzal FA, Rood JI, McClane BA (2013a) Toxin plasmids of Clostridium perfringens. Microbiol Mol Biol Rev 77(2):208–233
Li L, Shi Y, Cheserek MJ, Su G, Le G (2013b) Antibacterial activity and dual mechanisms of peptide analog derived from cell-penetrating peptide against Salmonella typhimurium and Streptococcus pyogenes. Appl Microbiol Biotechnol 97(4):1711–1723
Jiao J, Mao RY, Wang XM, Zhang Y, Teng D, Feng XJ, Wang JH (2015) GAP-initiated constitutive expression of a novel plectasin-derived peptide MP1106 by Pichia pastoris and its activity against Streptococcus suis. Process Biochem 50(2):253–261
Jones AM, Kuijper EJ, Wilcox MH (2013) Clostridium difficile: a European perspective. J Infect 66(2):115–128
Mygind PH, Fischer RL, Schnorr KM, Hansen MT, Sönksen CP, Ludvigsen S, Raventós D, Buskov S, Christensen B, De Maria L, Taboureau O, Yaver D, Elvig-Jørgensen SG, Sørensen MV, Christensen BE, Kjaerulff S, Frimodt-Moller N, Lehrer RI, Zasloff M, Kristensen HH (2005) Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 437(7061):975–980
Ngamwongsatit B, Tanomsridachchai W, Suthienkul O, Urairong S, Navasakuljinda W, Janvilisri T (2016) Multidrug resistance in Clostridium perfringens isolated from diarrheal neonatal piglets in Thailand. Anaerobe 38(2016):88–93
Paredes-Sabja D, Sarker MR (2009) Clostridium perfringens sporulation and its relevance to pathogenesis. Future Microbiol 4(5):519–525
Park M, Rooney AP, Hecht DW, Li J, McClane BA, Nayak R, Paine DD, Rafii F (2010) Phenotypic and genotypic characterization of tetracycline and minocycline resistance in Clostridium perfringens. Arch Microbiol 192(10):803–810
Powers JP, Hancock REW (2003) The relationship between peptide structure and antibacterial activity. Peptides 24(11):1681–1691
Rothan HA, Mohamed Z, Suhaeb AM, Rahman NA, Yusof R (2013) Antiviral cationic peptides as a strategy for innovation in global health therapeutics for denguevirus: high yield production of the biologically active recombinant plectasin peptide. OMICS 17(11):560–567
Schneider T, Kruse T, Wimmer R, Wiedemann I, Sass V, Pag U, Jansen A, Nielsen AK, Mygind PH, Raventós DS, Neve S, Ravn B, Bonvin AM, De Maria L, Andersen AS, Gammelgaard LK, Sahl HG, Kristensen HH (2010) Plectasin, a fungal defensin, targets the bacterial cell wall precursor Lipid II. Science 328(5982):1168–1172
Shah M, Bishburg E, Baran DA, Chan T (2009) Epidemiology and outcomes of clostridial bacteremia at a tertiary-care institution. Scientific World J 9:144–148
Silva RO, Lobato FC (2015) Clostridium perfringens: a review of enteric diseases in dogs, cats and wild animals. Anaerobe 33:14–17
Soge OO, Tivoli LD, Meschke JS, Roberts MC (2009) A conjugative macrolide resistance gene, mef(A), in environmental Clostridium perfringens carrying multiple macrolide and/or tetracycline resistance genes. J Appl Microbiol 106(1):34–40.
Teng D, Wang X, Xi D, Mao R, Zhang Y, Guan Q, Zhang J, Wang J (2014) A dual mechanism involved in membrane and nucleic acid disruption of AvBD103b, a new avian defensing from the king penguin, against Salmonella enteritidis CVCC3377. Appl Microbiol Biotechnol 98(19):8313–8325
Tripathi JK, Kathuria M, Kumar A, Mitra K, Ghosh JK (2015) An unprecedented alteration in mode of action of IsCT resulting its translocation into bacterial cytoplasm andinhibition of macromolecular syntheses. Sci Rep 5:9127
Tsuji BT, Rybak MJ (2006) Etest synergy testing of clinical isolates of Staphylococcus aureus demonstrating heterogeneous resistance to vancomycin. Diagn Microbiol Infect Dis 54(1):73–77
Uzal FA, Saputo J, Sayeed S, Vidal JE, Fisher DJ, Poon R, Adams V, Fernandez-Miyakawa ME, Rood JI, McClane BA (2009) Development and application of new mouse models to study the pathogenesis of Clostridium perfringens type C Enterotoxemias. Infect Immun 77(12):5291–5299
Uzal FA, McClane BA, Cheung JK, Theoret J, Garcia JP, Moore JR, Rood JI (2015) Animal models to study the pathogenesis of human and animal Clostridium perfringens infections. Vet Microbiol 179(1–2):23–33
Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R (2004) Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol 33(6):537–549
Xi D, Teng D, Wang X, Mao R, Yang Y, Xiang W, Wang J (2013) Design, expression and characterization of the hybrid antimicrobial peptide LHP7, connected by a flexible linker, against Staphylococcus and Streptococcus. Process Biochem 48(3):453–461
Zhang LX, Yan KZ, Zhang Y, Huang R, Bian J, Zheng CS, Sun HX, Chen ZH, Sun N, An R, Min FG, Zhao WB, Zhuo Y, You JL, Song YJ, Yu ZY, Liu ZH, Yang KQ, Gao H, Dai HQ, Zhang XL, Wang J, Fu CZ, Pei G, Liu JT, Zhang S, Goodfellow M, Jiang YY, Kuai J, Zhou GC, Chen XP (2007) High-throughput synergy screening identifies microbial metabolites as combination agents for the treatment of fungal infections. Proc Natl Acad Sci U S A 104(11):4606–4611
Zhang J, Yang Y, Teng D, Tian Z, Wang S, Wang J (2011) Expression of plectasin in Pichia pastoris and its characterization as a new antimicrobial peptide against Staphyloccocus and Streptococcus. Protein Expr Purif 78(2):189–196
Zhang Y, Teng D, Mao R, Wang X, Xi D, Hu X, Wang J (2014) High expression of a plectasin-derived peptide, NZ2114, in Pichia pastoris and its pharmacodynamics, postantibiotic and synergy against Staphylococcus aureus. Appl Microbiol Biotechnol 98(2):681–694
Zhang Y, Teng D, Wang X, Mao R, Cao X, Hu X, Zong L, Wang J (2015) In vitro and in vivo characterization of a new recombinant antimicrobial peptide, MP1102, against methicillin-resistant Staphylococcus aureus. Appl Microbiol Biotechnol 99(15):6255–6266
Acknowledgments
The authors acknowledged technicians Ms. Tong Zhao and Mr. Chunli Li from the Core Facility at the Institute of Microbiology at the Chinese Academy of Sciences (CAS) for their technical support with the flow cytometric and SEM analyses. This study was supported by the National Natural Science Foundation of China (No. 31572445, 31372346, 31572444 and 31302004), the Project of the National Support Program for Sci & Technol in China (No. 2013BAD10B02 and No. 2011BAD26 B02), the Special Fund for Agro-scientific Research in the Public Interest in China (No.201403047), the Basic Research Fund for Central Public Institutes in China (No.0032015030) and the AMP Direction of Innovation Program of Agric Sci & Tech in CAAS (CAAS-ASTIP-2013-FRI-02).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zong, L., Teng, D., Wang, X. et al. Mechanism of action of a novel recombinant peptide, MP1102, against Clostridium perfringens type C. Appl Microbiol Biotechnol 100, 5045–5057 (2016). https://doi.org/10.1007/s00253-016-7387-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7387-x