Abstract
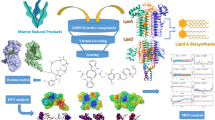
The main aim of the current study is to explore the bioactive potential of Streptomyces sp. VITJS8 isolated from the marine saltern. The cultural, biochemical, and morphological studies were performed to acquire the characteristic features of the potent isolate VITJS8. The 16Sr DNA sequencing was performed to investigate the phylogenetic relationship between the Streptomyces genera. The structure of the compound was elucidated by gas chromatography-mass spectrometry (GC-MS), infra-red (IR), and ultra-violet (UV) spectroscopic data analysis. The GC-MS showed the retention time at 22.39 with a single peak indicating the purity of the active compound, and the molecular formula was established as C14H9ONCl2 based on the peak at m/z 277 [M]+. Furthermore, separated by high-performance liquid chromatography (HPLC), their retention time (t r) 2.761 was observed with the absorption maxima at 310 nm. The active compound showed effective inhibitory potential against four clinical pathogens at 500 μg/mL. The antioxidant activity was found effective at the IC50 value of 500 μg/mL with 90 % inhibition. The 3-(4,5-dimethylthiazol-2-yl)-2,5-ditetrazolium bromide (MTT) assay revealed the cytotoxicity against HepG2 cells at IC50 of 250 μg/mL. The progression of apoptosis was evidenced by morphological changes by nuclear staining. The DNA fragmentation pattern was observed at 250 μg/mL concentration. Based on flow cytometric analysis, it was evident that the compound was effective in inhibiting the sub-G0/G1 phase of cell cycle. The in vitro findings were also supported by the binding mode molecular docking studies. The active compound revealed minimum binding energy of −7.84 and showed good affinity towards the active region of topoisomerase-2α that could be considered as a suitable inhibitor. Lastly, we performed 30 ns molecular dynamic simulation analysis using GROMACS to aid in better designing of anticancer drugs. Simulation result of root mean square deviation (RMSD) analysis showed that protein-ligand complex reaches equilibration state around 10 ns that illustrates the docked complex is stable. We propose the possible mechanism of sesquiterpenes to play a significant role in antitumor cascade. Hence, our studies open up a new facet for a potent drug as an anticancer agent.










Similar content being viewed by others
References
Alexander M (1977) Soil microbiology, 2nd edn. Wiley, New York, p 207
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSLBLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
American Cancer Society. Atlanta: cancer facts and figures, 2013. Available from URL:http://www.cancer.org
Arasu MV, Duraipandiyan V, Agastian P, Ignacimuthu S (2008) Antimicrobial activity of Streptomyces sp. ERI-26 recovered from Western Ghats of Tamil Nadu. J Mycol MeD 18:147–153
Balagurunathan R, Subramanian A (2001) Antagonistic Streptomycetes from marine sediments. Adv Bio Sc 20:71–6
Cho JY, Kwon HC, Williams PG, Jensen PR, Fenical W (2006) Azamerone, a terpenoidphthalazinone from a marine derived bacterium related to the genus Streptomyces (Actinomycetales). Org Lett 8:2471–2474
Covington RT (1988) Management of diarrhea. Drug News Lett 13:1–2
Demain AL (1999) Pharmaceutically active secondary metabolites of microorganisms. Appl Microbiol Biotechnol 52:455–463
Denning DW (2003) Echinocandin antifungal drugs. Lancet 362:1142–1151
Ding L, Pfoh R, Ruhl S, Qin S, Laatsch H (2009) T-muurololsesquiterpenes from the marine Streptomyces sp. M491 and revision of the configuration of previously reported amorphanes. J Nat Prod 72(1):99–101
Duraipandiyan V, Ignacimuthu S (2007) Antibacterial and antifungal activity of Cassia fistula L.: an ethnomedicinal plant. J Ethnopharmacol 112:590–594
Fenical W, Jensen PR (2006) Developing a new resource for drug discovery: marine actinomycete bacteria. Nat Chem Biol 2:666–673
Fenical W, Lobkovsky E, Cladry J (1999) Cyclomarins A-C, new antiinflammatory cyclic peptides produced by a marine bacterium (Streptomyces sp). J Am Chem Soc 21:11273–11276
Freshney RI, Hart E, Russell JM (1982) Isolation and purification of cell cultures from human tumors. In: Reid E, Cook GMW, Moore DJ (eds) (B): Biochemistry, vol 2. Horwood, Chicester
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Hardt IH, Jensen PR, Fenical (2000) Neomarinone, and new cytotoxic marinone derivatives, produced by a marine filamentous bacterium (Actinomycetales). Tetrahedron Lett 41:2073–2076
Hoeksema H, Smith CG (1961) Novobiocin. Prog Ind Microbiol 3:91–139
International Association for the Study of Pain, Epidemiology of Cancer Pain [Accessed March 25, 2013]. Available from URL: http://www.iasppain.org
Ji Z, Wang M, Zhang J, Wei S, Wu W (2007) Two new members of streptothricin class antibiotics from Streptomyces qinlingensis sp. nov. J Antibiot 60:739–744
Kerr KG, Snelling AM (2009) Pseudomonas aeruginosa: a formidable and ever present adversary. J Hosp Infect 73:338–344
Khalaf NA, Shakya AK, Al-Othman A, El-Agbar Z, Farah (2008) Antioxidant activity of some common plants. Turk J Biol 32:51–55
Kohler C, Orrenius S, Zhivotovsky B (2002) Evaluation of caspase activity in apoptotic cells. J Immunol Methods 265:97–110
Kutzner KJ (1986) The family Streptomycetaceae. In: Starr MP, Stolp H, Trper HG, Balows A, Schlegel HG (eds) The prokaryotes. A handbook on habitats, isolation, and identification of bacteria, vol 2. Springer, New York, pp 2028–2090
Li M, Miao ZH, Chen Z, Chen Q, Gui M, Lin LP, Sun P, Yi YH, Ding J (2010) Echinoside A, a new marine-derived anticancer saponin, targets topoisomerase 2 alpha by unique interference with its DNA binding and catalytic cycle. Ann Oncol 21(3):597–607
LodiseJr PTP, Kwa N, Graves A, Furuno J, Graffunder E, Lomaestro B, McGregor JC (2007) Predictors of 30-day mortality among patients with Pseudomonas aeruginosa blood stream infection: impact of delayed appropriate antibiotic selection. Antimicrob Agents Chemother 51:3510–3515
Michael M, Gottesman (2002) Mechanisms of cancer drug resistance. Annu Rev Med 53:615–27
Miller RE, Larkin JM (2009) Combination systemic therapy for advanced renal cell carcinoma. Oncology 4(12):1218–1224
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) Autodock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 16:2785–2791
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Motohashi K, Ueno R, Sue M, Furihata K, Matsumoto T, Dairi T, Omura S, Seto H (2007) Studies on terpenoids produced by actinomycetes: oxaloterpins A, B, C, D, and E, diterpenes from Streptomyces sp. KO-3988. J Nat Prod 70:1712–1717
Motohashi K, Sue M, Furihata K, Ito S, Seto H (2008) Terpenoids produced by actinomycetes: napyradiomycins from Streptomyces antimycoticus NT17. J Nat Prod 71(4):595–601
Mutai C, Bii C, Vagias C, Abatis D, Roussis V (2009) Antimicrobial activity of Acacia mellifera extracts and lupanetriterpenes. J Ethnopharmacol 123:143–148
Pandey B, Ghimire P, Agrawal VP (2004) Studies on the antimicrobial activity of actinomycetes isolated from Khumbu region of Nepal. Tribhuvan University, Kathmandu
Pathirana C, Jensen PR, Fenical W (1992) Marinone and debromomarinone: antibiotic sesquiterpenoidnaphthoquinones of a new structure class from a marine bacterium. Tetrahedron Lett 33:7663–7666
Patil TD, Thakare SV (2012) In silico evaluation of selected triterpene glycosides as a human DNA topoisomerase II alpha (α) inhibitor. Int J Pharm Pharm Sci 4(4):201–204
Pozarowski P, Darzynkiewicz Z (2004) Analysis of cell cycle by flow cytometry. Methods Mol Biol 281:301–311
Rainey FA, Ward-Rainey NL, Kroppenstedt RM, Tackebrandt E (1996) The genus Norcardiopsis represents a phylogenetically coherent taxon and a distinct actinomycetes lineage: proposal of Nocaridiopsaceae fam. nov. Int J Syst Bacteriol 46:1088–1092
Ravikumar YS, Mahadevan KM, Kumaraswamy MN, Vaidya VP, Manjunatha H, Kumar V, Satyanarayana ND (2008) Antioxidant, cytotoxic and genotoxic evaluation of alcoholic extract of Polyalthia cerasoides (Roxb.) Bedd. Environ Toxicol Pharmacol 26(2):142–146
Remya M, Vijayakumar R (2007) Isolation and characterization of marine antagonistic actinomycetes from West Coast of India. Med Biol 5:13–19
Sánchez-Alcázar JA, Ruíz-Cabello J, Hernández-Muñoz I, Pobre PS, de la Torre P, Siles-Rivas E, García I, Kaplan O, Muñoz-Yagüe MT, Solís-Herruzo JA (1997) Tumor necrosis factor-α increases ATP content in metabolically inhibited L929 cells preceding cell death. J Biol Chem 272(48):30167–30177
Schüttelkopf AW, Van Aalten DMF (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr Sect D: Biol Crystallogr 60(8):1355–1363
Shukla S, Srivastava RS, Shrivastava SK, Sodhi A, Kumar P (2012) Synthesis, molecular docking and biological evaluation of 4-cycloalkylidineamino 1,2-naphthoquinone semicarbazones as anticancer agents. Asian Pac J Tro Biomed 2(2):S1040–S1046
Silva JC, Rodrigues S, Feas X, Estevinho LM (2012) Antimicrobial activity, phenolic profile and role in the inflammation of propolis. Food ChemToxicol 50:1790–1795
Stritzke K, Schulz S, Laatsch H, Helmke E, Beil W (2004) Novel caprolactones from a marine Streptomycete. J Nat Prod 67:395–401
Taylor WR, Stark GR (2009) Regulation of the G2/M transition by p53. Oncogene 20:1803–1815
Van Gunsteren WF, Billeter SR, Eising AA, Hunenberger PH and Kruger P (1996) Biomolecular simulation: the GROMOS96 manual and user guide, vdf Hochschulverlag AG an der ETH Zurich and BIOMOS b.v, Zurich, Switzerland, pp 1–1042
Vermeulen K, VanBockstaele DR, Berneman ZN (2005) Apoptosis: mechanisms and relevance in cancer. Ann Hematol 84:627–639
Wendt KU, Schulz GE (1998) Isoprenoid biosynthesis: manifold chemistry catalyzed by similar enzymes. Structure 6:127–133
Wu SJ, Fotso S, Li F, Qin S, Kelter G, Fiebig HH, Laatsch H (2006) N-carboxamido-staurosporine and selina-4(14),7(11)-diene-8,9-diol, new metabolites from a marine Streptomyces sp. J Antibiot (Tokyo) 59(6):331–337
Xiang WS, Wang JD, Wang XL, Zhang J (2009) A novel macrolide compound from Streptomyces bingchenggensis: fermentation, isolation, structure elucidation and biological properties. J Antibiot 62:229–231
Zhao PJ, Li GH, Shen YM (2006) New chemical constituents from the endophyte Streptomyces species LR4612 cultivated on Maytenus hookeri. Chem Biodivers 3:337–342
Acknowledgments
The authors take this opportunity to thank the management of VIT University and CSIR for providing the facilities and encouragement to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 166 kb)
Rights and permissions
About this article
Cite this article
Naine, S.J., Devi, C.S., Mohanasrinivasan, V. et al. Binding and molecular dynamic studies of sesquiterpenes (2R-acetoxymethyl-1,3,3-trimethyl-4t-(3-methyl-2-buten-1-yl)-1t-cyclohexanol) derived from marine Streptomyces sp. VITJS8 as potential anticancer agent. Appl Microbiol Biotechnol 100, 2869–2882 (2016). https://doi.org/10.1007/s00253-015-7156-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7156-2