Abstract
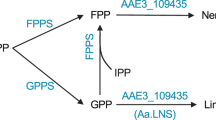
Lipases from the Candida rugosa-like family are enzymes with great biotechnological interest. In a previous work, several enzymes from this family were identified by in silico mining of fungal genomes. Here, we describe the cloning, expression, and characterization of putative lipases from the genomes of Nectria haematococca, Trichoderma reesei, and Aspergillus niger and compared their catalytic properties with those of OPE, a well-characterized sterol esterase/lipase from Ophiostoma piceae. All of them hydrolyzed p-nitrophenol esters and triglycerides with different efficiency, but their activity against sterol esters was dissimilar, and the enzyme from A. niger was unable of hydrolyzing these substrates while OPE showed the best k cat values, which in general leads to an improved catalytic efficiency. Similarly, OPE was the best catalyst in the synthesis of β-sitostanyl oleate, followed by the commercial CRL from C. rugosa, while the A. niger enzyme was unable to produce this compound. When the enzymes were evaluated for caprolactone oligomerization, the A. niger enzyme gave similar results than CRL, being OPE slightly more efficient. The expression of the putative selected proteins allowed their functional validation, suggesting that the hydrophobicity of the lid region may be an important factor, although the enzymatic efficiency is also influenced by other parameters, as the aggregation state and the size and morphology of the tunnel, where substrate recognition and catalysis takes place.





Similar content being viewed by others
References
Barba Cedillo V, Prieto A, Martínez AT, Martínez MJ (2011) Acylation procedure to obtain food and/or pharmaceutical compounds of interest using fungal sterol esterases. International patent ES 2395582:B1
Barba Cedillo V, Plou FJ, Martínez MJ (2012) Recombinant sterol esterase from Ophiostoma piceae: an improved biocatalyst expressed in Pichia pastoris. Microb Cell Factories 11:73
Barba Cedillo V, Prieto A, Martínez MJ (2013) Potential of Ophiostoma piceae sterol esterase for biotechnologically relevant hydrolysis reactions. Bioengineered 4:249–253
Barriuso J, Martínez MJ (2015) In silico metagenomes mining to discover novel esterases with industrial application by sequential search strategies. J Microbiol Biotechn 25:723–728
Barriuso J, Prieto A, Martínez MJ (2013) Fungal genomes mining to discover novel sterol esterases and lipases as catalysts. BMC Genomics 14:712
Bornscheuer UT (2002) Microbial carboxyl esterases: classification, properties and application in biocatalysis. FEMS Microbiol Rev 26:73–81
Brocca S, Secundo F, Ossola M, Alberghina L, Carrea G, Lotti M (2003) Sequence of the lid affects activity and specificity of Candida rugosa lipase isoenzymes. Protein Sci 12:2312–2319
Brush TS, Chapman R, Kurzman R, Williams DP (1999) Purification and characterization of extracellular lipases from Ophiostoma piliferum. Bioorgan Med Chem 7:2131–2138
Calero-Rueda O, Plou FJ, Ballesteros A, Martínez AT, Martínez MJ (2002a) Production, isolation and characterization of a sterol esterase from Ophiostoma piceae. Biochim Biophys Acta 1599:28–35
Calero-Rueda O, Gutiérrez A, del Río JC, Muñoz C, Plou FJ, Martínez AT, Martínez MJ (2002b) Method for the enzymatic control of pitch deposits formed during paper pulp production using an esterase that hydrolyses triglycerides and sterol esters. International patent WO 02/075045 A1R1:1–28
Calero-Rueda O, Gutiérrez A, del Río JC, Prieto A, Plou FJ, Ballesteros A, Martínez AT, Martínez MJ (2004) Hydrolysis of sterol esters by an esterase from Ophiostoma piceae: application for pitch control in pulping of Eucalyptus globulus wood. Intern J Biotechnol 6:367–375
Calero-Rueda O, Barba V, Rodríguez E, Plou F, Martínez ÁT, Martínez MJ (2009) Study of a sterol esterase secreted by Ophiostoma piceae: sequence, model and biochemical properties. BBA-Proteins Proteom 1794:1099–1106
Casas-Godoy L, Duquesne S, Bordes F, Sandoval G, Marty A (2012) Lipases: an overview. In: Sandoval, G (ed) Methods in molecular biology. Springer, New York, NY, 861:3–30
Diaz P, Prim N, Pastor FIJ (1999) Direct fluorescence-based lipase activity assay. Biotechniques 27:696–700
Domínguez de María P, Sánchez-Montero JM, Sinisterra JV, Alcántara AR (2006) Understanding Candida rugosa lipases: an overview. Biotechnol Adv 24:180–196
Gupta R, Kumari A, Syal P, Singh Y (2015) Molecular and functional diversity of yeast and fungal lipases: their role in biotechnology and cellular physiology. Prog Lipid Res 57:40–54
Gutiérrez A, del Río JC, Martínez MJ, Martínez AT (2001) The biotechnological control of pitch in paper pulp manufacturing. Trends Biotechnol 19:340–348
Gutierrez-Fernández J, Vaquero ME, Prieto A, Barriuso J, Martínez MJ, Hermoso JA (2014) Crystal structures of Ophiostoma piceae sterol esterase: structural insights into activation mechanism and product release. J Struct Biol 187:215–225
Hasan F, Shah AA, Hameed A (2006) Industrial applications of microbial lipases. Enzym Microb Technol 39:235–251
He WS, Jia CS, Ma YA, Yang YB, Zhang XM, Feng BA, Yue L (2010) Lipase-catalyzed synthesis of phytostanyl esters in non-aqueous media. J Mol Catal B-Enzym 67:60–65
Hyun J, Steinberg M, Treadwell CR, Vahouny GV (1971) Cholesterol esterase. A polymeric enzyme. Biochem Bioph Res Co 44:819–825
Jaeger KE, Eggert T (2002) Lipases for biotechnology. Curr Opin Biotechnol 13:390–397
Kobayashi S, Takeya K, Suda S, Uyama H (1998) Lipase-catalyzed ring-opening polymerization of medium-size lactones to polyesters. Macromol Chem Physic 199:1729–1736
Kontkanen H, Tenkanen M, Fagerström R, Reinikainen T (2004) Characterisation of steryl esterase activities in commercial lipase preparations. J Biotechnol 108:51–59
Kontkanen H, Tenkanen M, Reinikainen T (2006) Purification and characterisation of a novel steryl esterase from Melanocarpus albomyces. Enzym Microb Technol 39:265–273
Kumar A, Gross RA (2000) Candida antartica lipase B catalyzed polycaprolactone synthesis: effects of organic media and temperature. Biomacromolecules 1:133–138
López N, Pernas MA, Pastrana LM, Sánchez A, Valero F, Rúa ML (2004) Reactivity of pure Candida rugosa lipase isoenzymes (Lip1, Lip2, and Lip3) in aqueous and organic media. Influence of the isoenzymatic profile on the lipase performance in organic media. Biotechnol Progress 20:65–73
Mancheño JM, Pernas MA, Martínez MJ, Ochoa B, Rúa ML, Hermoso JA (2003) Structural insights into the lipase/esterase behavior in the Candida rugosa lipases family: crystal structure of the lipase 2 isoenzyme at 1.97 Å resolution. J Mol Biol 332:1059–1069
Musa-Veloso K, Poon TH, Elliot JA, Chung C (2011) A comparison of the LDL-cholesterol lowering efficacy of plant stanols and plant sterols over a continuous dose range: results of a meta-analysis of randomized, placebo-controlled trials. Prostag Leukotr Ess 85:9–28
Namekawa S, Suda S, Uyama H, Kobayashi S (1999) Lipase-catalyzed ring-opening polymerization of lactones to polyesters and its mechanistic aspects. Int J Biol Macromol 25:145–151
Norinobu S, Seo N, Sato F, Kaneko S, Mankura M (2003) Process for producing dietary sterol fatty acid esters. Patent US 6,660,491 B2
Pernas MA, López C, Rúa ML, Hermoso JA (2001) Influence of the conformational flexibility on the kinetics and dimerisation process of two Candida rugosa lipase isoenzymes. FEBS Lett 501:87–91
Pleiss J, Fischer M, Peiker M, Thiele C, Schmid RD (2000) Lipase engineering database: understanding and exploiting sequence-structure-function relationships. J Mol Catal B-Enzym 10:491–508
Plou FJ, Sogo P, Calvo M-V, Burguillo FJ, Ballesteros A (1997) Kinetic and enantioselective behaviour of isoenzymes A and B from Candida rugosa lipase in the hydrolysis of lipids and esters. Biocatal Biotransform 15:75–89
Saxena RK, Agarwal L, Meghwanshi GK (2005) Diversity of fungal and yeast lipases: present and future scenario for the 21th century. In: Satayanarayana T, Johri BN (eds) Microbial diversity; current perspectives and potential applications. I.K. International Publishing House Pvt ltd., New Delhi, pp. 791–814
Sinha J, Plantz BA, Inan M, Meagher MM (2005) Causes of proteolytic degradation of secreted recombinant proteins produced in methylotrophic yeast Pichia pastoris: case study with recombinant ovine interferon-τ. Biotechnol Bioeng 89:102–112
Teixeira ARS, Santos JLC, Crespo JG (2011) Production of steryl esters from vegetable oil deodorizer distillates by enzymatic esterification. Ind Eng Chem Res 50:2865–2875
Tenkanen M, Kontkanen H, Isoniemi R, Spetz P, Holmbom B (2002) Hydrolysis of steryl esters by a lipase (Lip 3) from Candida rugosa. Appl Microbiol Biotechnol 60:120–127
Uyama H, Kobayashi S (1993) Enzymatic ring-opening polymerization of lactones catalyzed by lipase. Chem Lett 7:1149–1150
Vaquero ME, Barriuso J, Medrano FJ, Prieto A, Martínez MJ (2015) Heterologous expression of a fungal sterol esterase/lipase in different hosts: effect on solubility, glycosylation and production. J Biosci Bioeng. doi:10.1016/j.jbiosc.2015.04.005
Varma IK, Albertsson AC, Rajkhowa R, Srivastava RK (2005) Enzyme catalyzed synthesis of polyesters. Prog Polym Sci 30:949–981
Weber N, Weitkamp P, Mukherjee KD (2001a) Fatty acid steryl, stanyl, and steroid esters by esterification and transesterification in vacuo using Candida rugosa lipase as catalyst. J Agr Food Chem 49:67–71
Weber N, Weitkamp P, Mukherjee KD (2001b) Steryl and stanyl esters of fatty acids by solvent-free esterification and transesterification in vacuo using lipases from Rhizomucor miehei, Candida antarctica, and Carica papaya. J Agr Food Chem 49:5210–5216
Acknowledgments
This work was supported by the Spanish projects BIO2012-36372, RTC-2014-1777-3, and S2013/MAE-2907. M.E. Vaquero gratefully acknowledges an FPU fellowship from MINECO. J. Barriuso thanks the financial support from the JAE-DOC CSIC program. The authors thank the help of the Proteomics (a member of ISCIII ProteoRed) and Analytical Ultracentrifugation CIB facilities, as well as to Dr. M. Hakkarainen for introducing M.E. Vaquero in polymerization reactions and Dr. F.J. Plou for his help in the pH-stat experiments.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(PDF 263 kb)
Rights and permissions
About this article
Cite this article
Vaquero, M.E., Prieto, A., Barriuso, J. et al. Expression and properties of three novel fungal lipases/sterol esterases predicted in silico: comparison with other enzymes of the Candida rugosa-like family. Appl Microbiol Biotechnol 99, 10057–10067 (2015). https://doi.org/10.1007/s00253-015-6890-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6890-9