Abstract
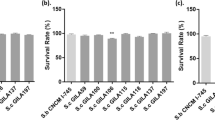
Epidermal growth factor (EGF) ameliorates stress and prevents incomplete gastrointestinal development in early-weaned piglets in commercial swine farming. This study aimed to further analyze the biological activities of intracellularly expressed EGF (IE-EGF), extracellularly expressed EGF (EE-EGF), and tagged EGF (T-EGF) from Saccharomyces cerevisiae in early-weaned pigs. In this study, we assigned 24 pigs to each of 5 groups that were provided a basic diet (the control group) or a diet supplemented with empty vector-expressing S. cerevisiae [the INVSc1(EV) group], T-EGF-expressing S. cerevisiae [the INVSc1-TE(−) group], EE-EGF-expressing S. cerevisiae [the INVSc1-EE(+) group], or IE-EGF-expressing S. cerevisiae [the INVSc1-IE(+) group]. All treatments were delivered at a dose of 60 μg EGF/kg body weight (BW) everyday. All the piglets were sacrificed after 21 day to determine their physio-biochemical indexes, immune functions, and intestinal development. In the piglet experiments, recombinant S. cerevisiae survived throughout the intestinal tract. The BW and intestinal development (e.g., mean villous height, crypt depth, villous height:crypt depth ratio (IVR), and total protein, DNA, and RNA contents) of the piglets were significantly enhanced in the INVSc1-IE(+) group compared with the animals in the INVSc1-EE(+) and INVSc1-TE(−) groups (P < 0.05). In addition, increased proliferating cell nuclear antigen (PCNA) staining was observed in the piglets that received the INVSc1-IE(+) treatment (approximately 80 %) compared with those that received the INVSc1-TE(−) (approximately 70 %) and INVSc1-EE(+) treatments (approximately 70 %). The levels of lactate dehydrogenase (LDH), creatine kinase (CK), alkaline phosphatase (ALP), immunoglobulin A (IgA), immunoglobulin M (IgM), and immunoglobulin G (IgG) were also significantly increased in the INVSc1-IE(+) group compared with the INVSc1-EE(+) and INVSc1-TE(−) groups (P < 0.05). Furthermore, the proliferation of piglet enterocytes was also significantly stimulated by both IE-EGF and EE-EGF compared with T-EGF in vitro (P < 0.05). Our data further demonstrate the previously reported hypothesis that IE-EGF is more suitable than EE-EGF or T-EGF for applications in early-weaned pigs.




Similar content being viewed by others
References
Akkermans ADL, Konstantinov SR, Zhu WY, Favier CF, Williams BA (2003) Postnatal development of the intestinal microbiota of the pig. In: Ball RO (ed) Proceedings of the 9th international symposium on digestive physiology in pigs. Banff, AB, Canada, pp 49-56
Bedford A, Li Z, Li M, Ji S, Liu W, Huai Y, Li J (2012) Epidermal growth factor-expressing Lactococcus lactis enhances growth performance of early-weaned pigs fed diets devoid of blood plasma. J Anim Sci 90:4–6. doi:10.2527/jas.53973
Bedford A, Huynh E, Fu M, Zhu C, Wey D, de Lange C, Li J (2014) Growth performance of early-weaned pigs is enhanced by feeding epidermal growth factor-expressing <i> Lactococcus lactis</i> fermentation product. J Biotechnol 173:47–52. doi:10.1016/j.jbiotec.2014.01.012
Buddington RK (1994) Nutrition and ontogenetic development of the intestine. Can J Physiol Pharmacol 72:251–259. doi:10.1139/y94-039
Chao JCJ, Liu KY, Chen SH, Fang CL, Tsao CW (2003) Effect of oral epidermal growth factor on mucosal healing in rats with duodenal ulcer. World J Gastroenterol 9:2261–2265
Cheung QC, Yuan Z, Dyce PW, Wu D, DeLange K, Li J (2009) Generation of epidermal growth factor-expressing Lactococcus lactis and its enhancement on intestinal development and growth of early-weaned mice. Am J Clin Nutr 89:871–879. doi:10.3945/ajcn.2008.27073
Chomczynski P (1993) A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 15:532–534
Corthesy B, Gaskins HR, Mercenier A (2007) Cross-talk between probiotic bacteria and the host immune system. J Nutr 137:S781–S790. doi:10.1146/annurev.mi.31.100177.000543
Donovan SM, Zijlstra RT, Odle J (1994) Use of the piglet to study the role of growth factors in neonatal intestinal development. Endocr Regul 28:153–162
Elliott SN, Wallace JL, McKnight W, Gall DG, Hardin JA, Olson M, Buret A (2000) Bacterial colonization and healing of gastric ulcers: the effects of epidermal growth factor. Am J Physiol Gastrointest Liver Physiol 278(1):G105–G112
Giang HH, Viet TQ, Ogle B, Lindberg JE (2010) Effects of different probiotic complexes of lactic acid bacteria on growth performance and gut environment of weaned piglets. Livest Sci 133:182–184. doi:10.1016/j.livsci.2010.06.059
Goldman AS, Atkinson SA, Hanson LA (1987) The effects of human milk on the recipient infant. Plenum Press, New York
Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM (2013) Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr 97:207–237. doi:10.1111/j.1439-0396.2012.01284.x
Kang P, Toms D, Yin Y, Cheung Q, Gong J, De Lange K, Li J (2010) Epidermal growth factor-expressing Lactococcus lactis enhances intestinal development of early-weaned pigs. J Nutr 140:806–811. doi:10.3945/jn.109.114173
Kiarie E, Bhandari S, Scott M, Krause DO, Nyachoti CM (2011) Growth performance and gastrointestinal microbial ecology responses of piglets receiving Saccharomyces cerevisiae fermentation products after an oral challenge with Escherichia coli (K88). J Anim Sci 89:1062–1078. doi:10.2527/jas. 2010-3424
Lackeyram D, Yang C, Archbold T, Swanson KC, Fan MZ (2010) Early weaning reduces small intestinal alkaline phosphatase expression in pigs. J Nutr 140(3):461–468. doi:10.3945/jn.109.117267
Lallès JP, Boudry G, Favier C, Le Floc’h N, Luron I, Montagne L, Sève B (2004) Gut function and dysfunction in young pigs: physiology. Anim Res 53:301–316. doi:10.1051/animres:2004018
Lallès JP, Bosi P, Smidt H, Stokes CR (2007) Weaning-a challenge to gut physiologists. Liv Sci 108:82–93. doi:10.1016/j.livsci.2007.01.091
Lamb-Rosteski JM, Kalischuk LD, Inglis GD, Buret AG (2008) Epidermal growth factor inhibits Campylobacter jejuni-induced claudin-4 disruption, loss of epithelial barrier function, and Escherichia coli translocation. Infect Immun 76:3390–3398. doi:10.1128/IAI. 01698-07
Le Bon M, Davies HE, Glynn C, Thompson C, Madden M, Wiseman J, Mellits KH (2010) Influence of probiotics on gut health in the weaned pig. Livest Sci 133:179–181. doi:10.1016/j.livsci.2010.06.058
Lee DL, Kuo TY, Chen MC, Tang TY, Liu FH, Weng CF (2006) Expression of porcine epidermal growth factor in Pichia pastoris and its biology activity in early-weaned piglets. Life Sci 78:649–654. doi:10.1016/j.lfs.2005.05.067
Lee DN, Chuang YS, Chiou HY, Wu FY, Yen HT, Weng CF (2007) Oral administration recombinant porcine epidermal growth factor enhances the jejunal digestive enzyme genes expression and activity of early-weaned piglets. J Anim Physiol Anim Nutr 92:463–470. doi:10.1111/j.1439-0396.2007.00735.x
Linher K, Wu D, Li J (2007) Glial cell line-derived neurotrophic factor: an intraovarian factor that enhances oocyte developmental competence in vitro. Endocrinology 148:4292–4301
Liu P, Piao XS, Thacker PA, Zeng ZK, Li PF, Wang D, Kim SW (2010) Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J Anim Sci 88:3871–3879. doi:10.2527/jas. 2009-2771
Marquardt RR (2011) Epidermal growth factor (EGF) and therapeutic antibodies for pig diets: an alternative approach to the use of antibiotics as growth promotants. Rev Comput Producciçon Porcina 18:177–180
Miettinen PJ, Perheentupa J, Otonkoski T, Lahteenmaki A, Panula P (1998) EGF-and TGF-alpha-like peptides in human fetal gut. Pediatr Res 26:25–30
NRC (2012) Nutrient requirements of swine, 11th edn. Natl Acad Press, Washington
O’Loughlin EV, Chung M, Hollenberg M, Hayden J, Zahavi I, Gall DG (1985) Effect of epidermal growth factor on ontogeny of the gastrointestinal tract. Am J Physiol 249:G674–G678
Odle J, Zijlstra RT, Donovan SM (1996) Intestinal effects of milkborne growth factors in neonates of agricultural importance. J Anim Sci 74:2509–2522
Pluske JR, Hampson DJ, Williams IH (1997) Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest Prod Sci 51:215–236. doi:10.1016/S0301-6226(97)00057-2
Price KL, Totty HR, Lee HB, Utt MD, Fitzner GE, Yoon I, Escobar J (2010) Use of Saccharomyces cerevisiae fermentation product on growth performance and microbiota of weaned pigs during Salmonella infection. J Anim Sci 88:3896–3908. doi:10.2527/jas. 2009-2728
Reid G, Jass J, Sebulsky MT, McCormick JK (2003) Potential uses of probiotics in clinical practice. Clin Microbiol Rev 16:658–672
Romanos, Scorer A, Clare JJ (1992) Foreign gene expression in yeast: a review. Yeast 8:423–488. doi:10.1002/yea.320080602
Savage DC (1997) Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31:107–133. doi:10.1146/annurev.mi.31.100177.000543
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17:741–754. doi:10.1016/S1521-6918(03)00052-0
Shen YB, Piao XS, Kim SW, Wang L, Liu P, Yoon I, Zhen YG (2009) Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J Anim Sci 87:2614–2624. doi:10.2527/jas. 2008-1512
Shirkey TW, Siggers RH, Goldade BG, Marshall JK, Drew MD, Laarveld B, Van Kessel AG (2006) Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp Biol Med (Maywood) 231:1333–1345
USDA (1991) National swine survey: morbidity/mortality and health management of swine in the U.S. National animal health monitoring system, animal and plant health inspection service, veterinary services. Washington, DC
Wang SJ, Zhou L, Chen HN, Cao YC, Zhang ZF, Yang JB, Huang YL, Guo CH (2014) Analysis of the biological activities of Saccharomyces cerevisiae expressing intracellular EGF, extracellular EGF, and tagged EGF in early-weaned rats. Appl Microbiol Biotechnol 1–11. doi:10.1007/s00253-014-6044-5
Wijtten PJ, Meulen JVD, Verstegen MW (2011) Intestinal barrier function and absorption in pigs after weaning: a review. Br J Nutr 105:967–981. doi:10.1017/S0007114510005660
Williams BA, Verstegen MWA, Tamminga S (2001) Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr Res Rev 14:207–227
Xu RJ, Wang F, Zhang SH (2000) Postnatal adaptation of the gastrointestinal tract in neonatal pigs: a possible role of milk-borne growth factors. Livest Prod Sci 66:95–107. doi:10.1016/S0301-6226(00)00217-7
Zanello G, Meurens F, Berri M, Chevaleyre C, Melo S, Auclair E, Salmon H (2011) Saccharomyces cerevisiae decreases inflammatory responses induced by F4 sup +sup enterotoxigenic Escherichia coli in porcine intestinal epithelial cells. Vet Immunol Immunopathol 141:133–138. doi:10.1016/j.vetimm.2011.01.018
Acknowledgments
We thank the teachers and workers at Shenzhen Premix Nutrition CO., LTD. This study was financially supported by the Ministry of Science and Technology of Sichuan Province (2012NZ0033) and Animal Science Discipline Program of Southwest University for Nationalities (2014XWD–S0905).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chunhua Guo and Lin Zhou contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, S., Guo, C., Zhou, L. et al. Comparison of the biological activities of Saccharomyces cerevisiae-expressed intracellular EGF, extracellular EGF, and tagged EGF in early-weaned pigs. Appl Microbiol Biotechnol 99, 7125–7135 (2015). https://doi.org/10.1007/s00253-015-6468-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6468-6