Abstract
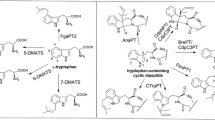
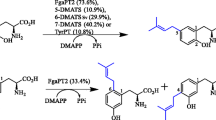
A putative prenyltransferase gene of the dimethylallyltryptophan synthase (DMATS) family, An13g01840, was identified in the genome sequence of Aspergillus niger. The deduced polypeptide CAK41583 consists of 465 amino acids with a calculated molecular mass of 52.7 kDa. To evaluate gene function, the coding sequence was cloned into pET28a and overexpressed in Escherichia coli. The soluble His6-fusion protein was purified to near homogeneity on Ni-NTA agarose and used for enzyme assays with diverse aromatic substrates in the presence of dimethylallyl diphosphate. HPLC analysis revealed product formation in the incubation mixtures with l-tyrosine and five derivatives thereof. Structure elucidation of the enzyme products by NMR and MS analyses confirmed O-prenylations and proved the identification of a tyrosine O-prenyltransferase (TyrPT). As in the case of SirD from Leptosphaeria maculans, TyrPT also accepted 4-amino-l-phenylalanine for an N-prenylation and l-tryptophan for a C7-prenylation. The K M values of TyrPT for l-tyrosine, l-tryptophan, and dimethylallyl diphosphate (DMAPP) were found to be 0.24, 0.19, and 0.71 mM, respectively. The k cat of l-tyrosine and l-tryptophan reactions were determined at 0.58 and 0.0053 s−1, respectively. The results presented in this study enhance the relationship of tyrosine O- and tryptophan C7-prenyltranferases and provide meanwhile a new enzyme for production of prenylated derivatives. In comparison to the known tyrosine prenyltransferase SirD, TyrPT showed significantly higher catalytic activity for several substrates, e.g., 4-amino-l-phenylalanine as well as 4- and 5-methyl-DL-tryptophan.



Similar content being viewed by others
References
Botta B, Vitali A, Menendez P, Misiti D, Delle MG (2005) Prenylated flavonoids: pharmacology and biotechnology. Curr Med Chem 12:717–739
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brakhage AA (2013) Regulation of fungal secondary metabolism. Nat Rev Microbiol 11:21–32
Gardiner DM, Cozijnsen AJ, Wilson LM, Pedras MS, Howlett BJ (2004) The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus Leptosphaeria maculans. Mol Microbiol 53:1307–1318
Heide L (2009) Prenyl transfer to aromatic substrates: genetics and enzymology. Curr Opin Chem Biol 13:171–179
Kremer A, Li S-M (2008) Potential of a 7-dimethylallyltryptophan synthase as a tool for production of prenylated indole derivatives. Appl Microbiol Biotechnol 79:951–961
Kremer A, Li S-M (2010) A tyrosine O-prenyltransferase catalyses the first pathway-specific step in the biosynthesis of sirodesmin PL. Microbiology 156:278–286
Kremer A, Westrich L, Li S-M (2007) A 7-dimethylallyltryptophan synthase from Aspergillus fumigatus: overproduction, purification and biochemical characterization. Microbiology 153:3409–3416
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li S-M (2009) Applications of dimethylallyltryptophan synthases and other indole prenyltransferases for structural modification of natural products. Appl Microbiol Biotechnol 84:631–639
Li S-M (2010) Prenylated indole derivatives from fungi: structure diversity, biological activities, biosynthesis and chemoenzymatic synthesis. Nat Prod Rep 27:57–78
Miyamoto K, Ishikawa F, Nakamura S, Hayashi Y, Nakanishi I, Kakeya H (2014) A 7-dimethylallyl tryptophan synthase from a fungal Neosartorya sp.: biochemical characterization and structural insight into the regioselective prenylation. Bioorg Med Chem 22:2517–2528
Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K, Andersen MR, Bendtsen JD, Benen JA, van den Berg M, Breestraat S, Caddick MX, Contreras R, Cornell M, Coutinho PM, Danchin EG, Debets AJ, Dekker P, van Dijck PW, van Dijk A, Dijkhuizen L, Driessen AJ, d'Enfert C, Geysens S, Goosen C, Groot GS, de Groot PW, Guillemette T, Henrissat B, Herweijer M, van den Hombergh JP, van den Hondel CA, van der Heijden RT, van der Kaaij RM, Klis FM, Kools HJ, Kubicek CP, van Kuyk PA, Lauber J, Lu X, van der Maarel MJ, Meulenberg R, Menke H, Mortimer MA, Nielsen J, Oliver SG, Olsthoorn M, Pal K, van Peij NN, Ram AF, Rinas U, Roubos JA, Sagt CM, Schmoll M, Sun J, Ussery D, Varga J, Vervecken W, van de Vondervoort PJ, Wedler H, Wösten HA, Zeng AP, van Ooyen AJ, Visser J, Stam H (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25:221–231
Rudolf JD, Poulter CD (2013) Tyrosine O-prenyltransferase SirD catalyzes S-, C-, and N-prenylations on tyrosine and tryptophan derivatives. ACS Chem Biol 8:2707–2714
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Steffan N, Li S-M (2009) Increasing structure diversity of prenylated diketopiperazine derivatives by using a 4-dimethylallyltryptophan synthase. Arch Microbiol 191:461–466
Steffan N, Unsöld IA, Li S-M (2007) Chemoenzymatic synthesis of prenylated indole derivatives by using a 4-dimethylallyltryptophan synthase from Aspergillus fumigatus. Chembiochem 8:1298–1307
Steffan N, Grundmann A, Yin W-B, Kremer A, Li S-M (2009) Indole prenyltransferases from fungi: a new enzyme group with high potential for the production of prenylated indole derivatives. Curr Med Chem 16:218–231
Winkelblech J, Li S-M (2014) Biochemical investigations of two 6-DMATS enzymes from Streptomyces revealing novel features of L-tryptophan prenyltransferases. Chembiochem 15:1030–1039
Wollinsky B, Ludwig L, Hamacher A, Yu X, Kassack MU, Li S-M (2012a) Prenylation at the indole ring leads to a significant increase of cytotoxicity of tryptophan-containing cyclic dipeptides. Bioorg Med Chem Lett 22:3866–3869
Wollinsky B, Ludwig L, Xie X, Li S-M (2012b) Breaking the regioselectivity of indole prenyltransferases: identification of regular C3-prenylated hexahydropyrrolo[2,3-b]indoles as side products of the regular C2-prenyltransferase FtmPT1. Org Biomol Chem 10:9262–9270
Woodside AB, Huang Z, Poulter CD (1988) Trisammonium geranyl diphosphate. Org Synth 66:211–215
Yin W-B, Baccile JA, Bok JW, Chen Y, Keller NP, Schroeder FC (2013) A nonribosomal peptide synthetase-derived iron(III) complex from the pathogenic fungus Aspergillus fumigatus. J Am Chem Soc 135:2064–2067
Yu X, Li S-M (2012) Prenyltransferases of the dimethylallyltryptophan synthase superfamily. Methods Enzymol 516:259–278
Yu X, Liu Y, Xie X, Zheng X-D, Li S-M (2012) Biochemical characterization of indole prenyltransferases: filling the last gap of prenylation positions by a 5-dimethylallyltryptophan synthase from Aspergillus clavatus. J Biol Chem 287:1371–1380
Zou H-X, Xie X, Zheng X-D, Li S-M (2011) The tyrosine O-prenyltransferase SirD catalyzes O-, N-, and C-prenylations. Appl Microbiol Biotechnol 89:1443–1451
Acknowledgments
We thank Prof. Michael Müller (Freiburg, Germany) for providing the A. niger strain, Lena Ludwig for synthesis of DMAPP, and Nina Zitzer and Stefan Newel for taking MS and NMR spectra, respectively. This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (Li844/4-1 to S.-M. L.) and by a PPP program of Deutscher Akademischer Austauschdienst and China scholarship council (to S.-M. L. and H.X.). Aili Fan is a recipient of a scholarship from China scholarship council.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1521 kb)
Rights and permissions
About this article
Cite this article
Fan, A., Chen, H., Wu, R. et al. A new member of the DMATS superfamily from Aspergillus niger catalyzes prenylations of both tyrosine and tryptophan derivatives. Appl Microbiol Biotechnol 98, 10119–10129 (2014). https://doi.org/10.1007/s00253-014-5872-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5872-7