Abstract
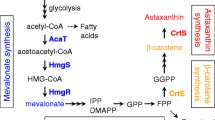
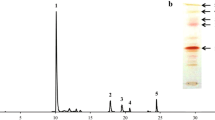
Astaxanthin is a valuable carotenoid that is widely used in the aquaculture, food, pharmaceutical, and cosmetic industries. Xanthophyllomyces dendrorhous is a carotenoid-synthesizing yeast strain that produces astaxanthin as its main pigment. Although metabolic engineering using gene manipulation is a valuable way to improve astaxanthin production, a gene expression system for X. dendrorhous has been poorly developed. In this study, three known promoters of X. dendrorhous, glycerol-3-phosphate dehydrogenase (gpd) promoter (Pgpd), glucose dehydrogenase (gdh) promoter (Pgdh), and actin (act) promoter (Pact), were evaluated for use in the overexpression of target proteins using green fluorescence protein (GFP) as an expression level indicator protein. The actin promoter, Pact, showed the highest expression level of GFP when compared with Pgpd and Pgdh. Additionally, to obtain new promoters for higher expression of target protein in X. dendrorhous, intracellular GFP intensity was evaluated for 13 candidate promoters. An alcohol dehydrogenase promoter, Padh4, showed more efficient expression of GFP rather than Pact. Overexpression of crtE gene encoding rate-limiting enzyme of carotenoid synthesis under the adh4 promoter yielded an increase in intracellular astaxanthin content of about 1.7-fold compared with the control strain. The promoters identified in this study must be useful for improving carotenoids production in X. dendrorhous.





Similar content being viewed by others
References
Breitenbach J, Visser H, Verdoes JC, van Ooyen AJ, Sandmann G (2011) Engineering of geranylgeranyl pyrophosphate synthase levels and physiological conditions for enhanced carotenoid and astaxanthin synthesis in Xanthophyllomyces dendrorhous. Biotechnol Lett 33:755–761
Gatignol A, Dassain M, Tiraby G (1990) Cloning of Saccharomyces cerevisiae promoters using a probe vector based on phleomycin resistance. Gene 91:35–41
Gu WL, An GH, Johnson EA (1997) Ethanol increases carotenoid production in Phaffia rhodozyma. J Ind Microbiol Biotechnol 19:114–117
Melillo E, Setroikromo R, Quax WJ, Kayser O (2013) Production of α-cuprenene in Xanthophyllomyces dendrorhous: a step closer to a potent terpene biofactory. Microb Cell Fact 12:13
Miao L, Chi S, Tang Y, Su Z, Yin T, Guan G, Li Y (2011) Astaxanthin biosynthesis is enhanced by high carotenogenic gene expression and decrease of fatty acids and ergosterol in a Phaffia rhodozyma mutant strain. FEMS Yeast Res 11:192–201
Nghiem NP, Kim TH, Yoo CG, Hicks KB (2013) Enzymatic fractionation of SAA-pretreated barley straw for production of fuel ethanol and astaxanthin as a value-added co-product. Appl Biochem Biotechnol 171:341–351
Niklitschek M, Alcaíno J, Barahona S, Sepúlveda D, Lozano C, Carmona M, Marcoleta A, Martínez C, Lodato P, Baeza M, Cifuentes V (2008) Genomic organization of the structural genes controlling the astaxanthin biosynthesis pathway of Xanthophyllomyces dendrorhous. Biol Res 41:93–108
Partow S, Siewers V, Bjørn S, Nielsen J, Maury J (2010) Characterization of different promoters for designing a new expression vector in Saccharomyces cerevisiae. Yeast 27:955–964
Rodríguez-Sáiz M, Godio RP, Alvarez V, de la Fuente JL, Martín JF, Barredo JL (2009) The NADP-dependent glutamate dehydrogenase gene from the astaxanthin producer Xanthophyllomyces dendrorhous: use of its promoter for controlled gene expression. Mol Biotechnol 41:165–172
Rodríguez-Sáiz M, de la Fuente JL, Barredo JL (2010) Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl Microbiol Biotechnol 88:645–658
Satoh A, Tsuji S, Okada Y, Murakami N, Urami M, Nakagawa K, Ishikura M, Katagiri M, Koga Y, Shirasawa T (2009) Preliminary clinical evaluation of toxicity and efficacy of a new astaxanthin-rich Haematococcus pluvialis extract. J Clin Biochem Nutr 44:280–284
Schmidt I, Schewe H, Gassel S, Jin C, Buckingham J, Hümbelin M, Sandmann G, Schrader J (2011) Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 89:555–571
Slaninová I, Kucsera J, Svoboda A (1999) Topology of microtubules and actin in the life cycle of Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Antonie Van Leeuwenhoek 75:361–368
Tripathi DN, Jena GB (2009) Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: a study in mice. Chem Biol Interact 80:398–406
Verdoes JC, Sandmann G, Visser H, Diaz M, van Mossel M, van Ooyen AJ (2003) Metabolic engineering of the carotenoid biosynthetic pathway in the yeast Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Appl Environ Microbiol 69:3728–3738
Wery J, Gutker D, Renniers AC, Verdoes JC, van Ooyen AJ (1997) High copy number integration into the ribosomal DNA of the yeast Phaffia rhodozyma. Gene 184:89–97
Young T, Williamson V, Taguchi A, Smith M, Sledziewski A, Russell D, Osterman J, Denis C, Cox D, Beier D (1982) The alcohol dehydrogenase genes of the yeast, Saccharomyces cerevisiae: isolation, structure, and regulation. Basic Life Sci 19:335–361
Acknowledgments
This study was supported by the Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction Kobe, iBioK), MEXT, Japan. Hara KY was supported by a Grant-in-Aid for Young Scientists (B) (22760608).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kiyotaka Y. Hara and Toshihiko Morita contributed equally in this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 38 kb)
Rights and permissions
About this article
Cite this article
Hara, K.Y., Morita, T., Endo, Y. et al. Evaluation and screening of efficient promoters to improve astaxanthin production in Xanthophyllomyces dendrorhous . Appl Microbiol Biotechnol 98, 6787–6793 (2014). https://doi.org/10.1007/s00253-014-5727-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5727-2