Abstract
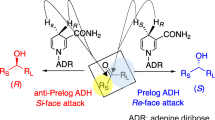
The asymmetric reduction of ketones is one of the most promising processes for producing chiral alcohols. However, dehydrogenases or reductases that can catalyze the reduction of ketones to give anti-Prelog chiral alcohols have been limited to some NADP+/NADPH-dependent enzymes. Recently, we reported a novel NAD+/NADH-dependent alcohol dehydrogenase (ADH) from Leifsonia sp. and Pseudomonas ADH homologs from soil metagenomes. Moreover, we have established an efficient hydrogen-transfer bioreduction process with 2-propanol as a hydrogen donor using Leifsonia ADH. This review focuses on the recent development of novel ADHs for producing industrially useful anti-Prelog chiral alcohols from various ketones.








Similar content being viewed by others
References
Asako H, Wakita R, Matsumura K, Shimizu M, Sakai J, Itoh N (2005) Purification and cDNA cloning of NADPH-dependent aldoketoreductase, involved in asymmetric reduction of methyl 4-bromo-3-oxobutyrate, from Penicillium citrinum IFO4631. Appl Environ Microbiol 71:1101–1104
Asako H, Shimizu M, Itoh N (2008) Engineering of NADPH-dependent aldo-keto reductase from Penicillium citrinum by directed evolution to improve thermostability and enantioselectivity. Appl Microbiol Biotechnol 80:805–812
Bahulekar R, Ayyangar NR, Ponrathnam S (1991) Polyethyleneimine in immobilization of biocatalysts. Enzyme Microb Technol 13:858–868
Bradshaw CW, Fu H, Shen GJ, Wong CH (1992b) A Pseudomonas sp. alcohol dehydrogenase with broad substrate specificity and unusual stereospecificity for organic synthesis. J Org Chem 57:1526–1532
Bradshow CW, Hummel W, Wong CH (1992a) Lactobacillus kefir alcohol dehydrogenase: a useful catalyst for synthesis. J Org Chem 57:1532–1536
Ehrensberger AH, Elling RA, Wilson DK (2006) Structure-guided engineering of xylitol dehydrogenase cosubstrate specificity. Structure 14:567–575
Feil IK, Hendle J, Schomburg D (1997) Modified substrate specificity of L-hydroxyisocaproate dehydrogenase derived from structure-based protein engineering. Protein Eng 10:255–262
Hu J, Xu Y (2006) Anti-Prelog reduction of prochiral carbonyl compounds by Oenococcus oeni in a biphasic system. Biotechnol Lett 28:1115–1119
Huisman GW, Liang J, Krebber A (2010) Practical chiral alcohol manufacture using ketoreductases. Curr Opin Chem Biol 14:1–8
Hummel W (1997) New alcohol dehydrogenases for the synthesis of chiral compounds. Adv Biochem Eng Biotechnol 58:145–184
Hummel W, Kula MR (1989) Dehydrogenases for the synthesis of chiral compounds. Eur J Biochem 184:1–13
Inoue K, Makino Y, Itoh N (2005a) Purification and characterization of a novel alcohol dehydrogenase from Leifsonia sp. strain S749: a promising biocatalyst for an asymmetric hydrogen transfer bioreduction. Appl Environ Microbiol 71:3633–3641
Inoue K, Makino Y, Itoh N (2005b) Production of (R)-chiral alcohols by a hydrogen-transfer bioreduction with NADH-dependent Leifsonia alcohol dehydrogenase (LSADH). Tetrahedron Asymmetry 16:2539–2549
Inoue K, Makino Y, Dairi T, Itoh N (2006) Gene cloning and expression of Leifsonia alcohol dehydrogenase (LSADH) involved in asymmetric hydrogen-transfer bioreduction to produce (R)-form chiral alcohols. Biosci Biotechnol Biochem 70:418–426
Isotani K, Kurokawa J, Itoh N (2012) Production of (R)-3-quinuclidinol by E. coli biocatalysts possessing NADH-dependent 3-quinuclidinone reductase (QNR or bacC) from Microbacterium luteolum and Leifsonia alcohol dehydrogenase (LSADH). Int J Mol Sci 13:13542–13553
Itoh N, Makino Y (2013) Protein engineering: development of novel enzymes for the improved reduction of C = O double bonds. In: Brenna E (ed) Synthetic methods for biologically active molecules. Wiley, Weinheim, pp 139–185
Itoh N, Matsuda M, Mabuchi M, Dairi T, Wang J (2002) Chiral alcohol production by NADH-dependent phenylacetaldehyde reductase coupled with in situ regeneration of NADH. Eur J Biochem 269:2394–2402
Itoh N, Nakamura M, Inoue K, Makino Y (2007) Continuous production of chiral 1,3-butanediol using immobilized biocatalysts in a packed bed reactor: promising biocatalysis method with an asymmetric hydrogen-transfer bioreduction. Appl Microbiol Biotechnol 75:1249–1256
Itoh N, Isotani K, Nakamura M, Inoue K, Isogai Y, Makino Y (2012) Efficient synthesis of optically pure alcohols by asymmetric hydrogen-transfer biocatalysis: application of engineered enzymes in a 2-propanol-water medium. Appl Microbiol Biotechnol 93:1075–1085
Itoh N, Isotani K, Makino Y, Kato M, Kitayama K, Ishimota T (2014) PCR-based amplification and heterologous expression of Pseudomonas alcohol dehydrogenase genes from the soil metagenome for biocatalysis. Enzyme Microb Technol 50:140–150
Jakoblinnert A, Mladenov R, Paul A, Sibilla F, Schwaneberg U, Ansorge-Schumacher MB, de Domı´nguez MP (2011) Asymmetric reduction of ketones with recombinant E. coli whole cells in neat substrates. Chem Commun 47:12230–12232
Jörnvall H, Hedlund J, Bergman T, Oppermann U, Persson B (2010) Superfamilies SDR and MDR: from early ancestry to present forms. Emergence of three lines, a Zn-metalloenzyme, and distinct variabilities. Biochem Biophys Res Commun 396:125–130
Kaluzna IA, Matsuda T, Sewell AK, Stewart JD (2004) Systematic investigation of Saccharomyces cerevisiae enzymes catalyzing carbonyl reductions. J Am Chem Soc 126:12827–12832
Kaluzna IA, Feske BD, Wittayanan W, Ghiviriga I, Stewart JD (2005) Stereoselective, biocatalytic reductions of α-chloro-β-keto esters. J Org Chem 70:342–345
Kara S, Schrittwieser JH, Hollmann F (2013) Strategies for cofactor regeneration in biocatalyzed reductions. In:Brenna E (ed) Synthetic methods for biologically active molecules. Wiley, Weinheim, pp 209–238
Kita K, Fukura T, Nakase KI, Okamoto K, Yanase H, Kataoka M, Shimizu S (1999a) Cloning, overexpression, and mutagenesis of the Sporobolomyces salmonicolor AKU4429 gene encoding a new aldehyde reductase, which catalyzes the stereoselective reduction of ethyl 4-chloro-3-oxobutanoate to ethyl (S)-4-chloro-3-hydro xybutanoate. Appl Environ Microbiol 65:5207–5211
Kita K, Nakase K, Yanase H, Kataoka M, Shimizu S (1999b) Purification and characterization of new aldehyde reductases from Sporobolomyces salmonicolor AKU4429. J Mol Catal B Enzym 6:305–313
Knietsch A, Waschkowitz T, Bowien S, Henne A, Daniel R (2003a) Construction and screening of metagenomic libraries derived from enrichment cultures: generation of a gene bank for genes conferring alcohol oxidoreductase activity on Escherichia coli. Appl Environ Microbiol 69:1408–1416
Knietsch A, Bowien S, Whited G, Gottschalk G, Daniel R (2003b) Identification and characterization of coenzyme B12-dependent glycerol dehydratase- and diol dehydratase-encoding genes from metagenomic DNA libraries derived from enrichment cultures. Appl Environ Microbiol 69:3048–3060
Kroutil W, Mang H, Edegger K, Faber K (2004) Recent advance in the biocatalytic reduction of ketones and oxidation of sec-alcohols. Curr Opin Chem Biol 8:120–126
Kubota M, Nodate M, Yasumoto-Hirose M, Uchiyama T, Kagami O, Shizuri Y, Misawa N (2005) Isolation and functional analysis of cytochrome P450 CYP153A genes from various environments. Biosci Biotechnol Biochem 69:2421–2430
Lamed RJ, Keinan E, Zeikus JG (1981) Potential applications of an alcohol-aldehyde/ketone oxidoreductase from thermophilic bacteria. Enzyme Mircob Technol 3:144–148
Lefevre F, Jarrin C, Ginolhac A, Auriol D, Nalin R (2007) Environmental metagenomics: an innovative resource for industrial biocatalysis. Biocatal Biotrans 25:242–250
Lorenz P, Eck J (2005) Metagenomics and industrial applications. Nature 3:510–516
Makino Y, Dairi T, Itoh N (2007) Engineering the phenylacetaldehyde reductase mutant for improved substrate conversion in the presence of concentrated 2-propanol. Appl Microbiol Biotechnol 77:833–843
Martín-Matute B, Edin M, Bogár K, Kaynak FB, Bäckvall JE (2005) Combined ruthenium(II) and lipase catalysis for efficient dynamic kinetic resolution of secondary alcohol: insight into the racemization mechanism. J Am Chem Soc 127:8817–8825
Matsuda T, Yamanaka R, Nakamura K (2009) Recent progress in biocatalysis for asymmetric oxidation and reduction. Tetrahedron Asymmetry 20:513–557
Matsuyama A, Yamamoto H, Kobayashi Y (2002) Practical application of recombinant whole-cell biocatalysts for the manufacturing of pharmaceutical such as chiral alcohols. Org Process Res Dev 6:558–561
Meng F, Xu Y (2010) Purification and characterization of an anti-Prelog alcohol dehydrogenase from Oenococcus oeni that reduces 2-octanone to (R)-2-octanol. Biotechnol Lett 32:533–537
Moon HJ, Tiwar MK, Singh R, Kang YC, Lee JK (2012) Molecular determinants of the cofactor specificity of ribitol dehydrogenase, a short-chain dehydrogenase/reductase. Appl Environ Microbiol 78:3079–3086
Morikawa S, Nakai T, Yasohara Y, Namba H, Kizaki N, Hasegawa J (2005) Highly active mutants of carbonyl reductase S1 with inverted coenzyme specificity and production of optically active alcohols. Biosci Biotechnol Biochem 69:544–552
Nie Y, Xu Y, Mu XQ, Wang HY, Yang M, Xiao R (2007) Purification, characterization, gene cloning, and expression of a novel alcohol dehydrogenase with anti-Prelog stereospecificity from Candida parapsilosis. Appl Environ Microbiol 73:3759–3764
Noyori R (1994) Asymmetric catalysis in organic synthesis. Wiley, New York
Noyori R, Ohkuma T (2001) Asymmetric catalysis by architectural and functional molecular engineering: practical chemo- and stereoselective hydrogenation of ketones. Angew Chem Int Ed 40:40–73
Persson B, Kallberg Y, Bray JE, Bruford E, Dellaporta SL, Favia AD, Duarte RG, Jörnvall H, Kavanagh KL, Kedishvili N, Kisiela M, Maser E, Mindnich R, Orchard S, Penning TM, Thornton JM, Adamski J, Oppermann U (2009) The SDR (short-chain dehydrogenase/reductase and related enzymes) nomenclature initiative. Chemico-Biol Interact 178:94–98
Prelog V (1964) Specification of the stereospecificity of some oxidoreductases by diamond lattice sections. Pure Appl Chem 9:119–130
Rocha-Martin J, Vega D, Bolivar JM, Hidalgo A, Berenguer J, Guisan JM, Lopez-Gallego F (2012) Characterization and further stabilization of a new anti-Prelog specific alcohol dehydrogenase from Thermus thermophilus HB27 for asymmetric reduction of carbonyl compounds. Bioresour Technol 103:343–350
Schlieben NH, Niefind K, Müller J, Riebel B, Hummel W, Schomburg D (2005) Atomic resolution structures of R-specific alcohol dehydrogenase from Lactobacillus brevis provide the structural bases of its substrate and cosubstrate specificity. J Mol Biol 349: 801–813
Stemmer WP (1994) Rapid evolution of a protein in vitro by DNA shuffling. Nature 370:389–391
Tanaka N, Nonaka T, Nakanishi M, Deyashiki Y, Hara A, Mitsui Y (1996) Crystal structure of the ternary complex of mouse lung carbonyl reductase at 1.8 Å resolution: the structural origin of coenzyme specificity in the short-chain dehydrogenase/reductase family. Structure 4:33–45
Tasnádi G, Hall M (2013) Relevant practical applications of bioreduction processes in the synthesis of active pharmaceutical ingredients. In: Brenna E (ed) Synthetic methods for biologically active molecules. Wiley, Weinheim, pp 329–374
Toda H, Imae R, Itoh N (2012) Efficient biocatalysis for the production of enantiopure (S)-epoxides using styrene monooxygenase (SMO) and Leifsonia alcohol dehydrogenase (LSADH) system. Tetrahedron Asymmetry 23:1542–1549
Uchiyama T, Miyazaki K (2010) Product-induced gene expression, a product-responsive reporter assay used to screen metagenomic libraries for enzyme-encoding gene. Appl Environ Microbiol 76:7029–7035
van Hellemond EW, Janssen DB, Fraaije MW (2007) Discovery of a novel styrene monooxygenase originating from the metagenome. Appl Environ Microbiol 73:5832–5839
Wada M, Kataoka M, Kawabata H, Yasohara Y, Kizaki N, Hasegawa J, Shimizu S (1998) Purification and characterization of NADPH-dependent carbonyl reductase, involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate, from Candida magnoliae. Biosci Biotechnol Biochem 62:280–285
Wallner SR, Lavandera I, Mayer SF, Ohrlein R, Hafner A, Edegger K, Faber K, Kroutil W (2008) Stereoselective anti-Prelog reduction of ketones by whole cells of Comamonas testosteroni in a ‘substrate-coupled’ approach. J Mol Catal B Enzym 55:126–129
Wang Q, Wu H, Wang A, Du P, Pei X, Li H, Yin X, Huang L, Xiong X (2010) Prospecting metagenomic enzyme subfamily genes for DNA family shuffling by a novel PCR-based approach. J Biol Chem 285:41509–41516
Weckbecker A, Hummel W (2006) Cloning, expression, and characterization of an (R)-specific alcohol dehydrogenase from Lactobacillus kefir. Biocatal Biotrans 24:380–389
Weinhold EG, Benner SA (1995) Engineering yeast alcohol dehydrogenase: replacing Trp54 by Leu broadens substrate specificity. Protein Eng Des Sel 8:457–461
Wong CH, Whitesides G (1994) Enzymes in synthetic organic chemistry. Pergamon, Oxford
Yasohara Y, Kizaki N, Hasegawa S, Takahashi S, Wada M, Kataoka M, Shimizu S (2000) Molecular cloning and overexpression of the gene encoding an NADPH-dependent carbonyl reductase from Candida magnoliae, involved in stereoselective reduction of ethyl 4-chloro-3-oxobutanoate. Biosci Biotechnol Biochem 64:1430–1436
Ying X, Ma K (2011) Characterization of a zinc-containing alcohol dehydrogenase with stereoselectivity from the hyperthermophilic archaeon Thermococcus guaymasensis. J Bacteriol 193:3009–3019
Yun J, Kang S, Park S, Yoon H, Kim MJ, Heu S, Ryu S (2004) Characterization of a novel amylolytic enzyme encoded by a gene from soil-derived metagenomic library. Appl Environ Microbiol 70:7229–7235
Zhang R, Zhu G, Zhang W, Cao S, Ou X, Li X, Bartlam M, Xu Y, Zhang XC, Rao Z (2008) Crystal structure of a carbonyl reductase from Candida parapsilosis with anti-Prelog stereospecificity. Protein Sci 17:1412–1423
Zhang R, Geng Y, Xu Y, Zhang W, Wang S, Xiao R (2011) Carbonyl reductase SCRII from Candida parapsilosis catalyzes anti-Prelog reaction to (S)-1-phenyl-1,2-ethanediol with absolute stereochemical selectivity. Bioresour Technol 102:483–489
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Itoh, N. Use of the anti-Prelog stereospecific alcohol dehydrogenase from Leifsonia and Pseudomonas for producing chiral alcohols. Appl Microbiol Biotechnol 98, 3889–3904 (2014). https://doi.org/10.1007/s00253-014-5619-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5619-5