Abstract
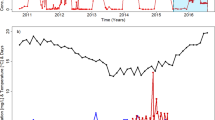
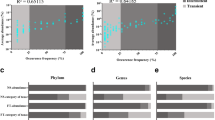
Wastewater treatment plants use a variety of bioreactor types and configurations to remove organic matter and nutrients. Little is known regarding the effects of different configurations and within-plant immigration on microbial community dynamics. Previously, we found that the structure of ammonia-oxidizing bacterial (AOB) communities in a full-scale dispersed growth activated sludge bioreactor correlated strongly with levels of NO2 − entering the reactor from an upstream trickling filter. Here, to further examine this puzzling association, we profile within-plant microbial biogeography (spatial variation) and test the hypothesis that substantial microbial immigration occurs along a transect (raw influent, trickling filter biofilm, trickling filter effluent, and activated sludge) at the same full-scale wastewater treatment plant. AOB amoA gene abundance increased >30-fold between influent and trickling filter effluent concomitant with NO2 − production, indicating unexpected growth and activity of AOB within the trickling filter. Nitrosomonas europaea was the dominant AOB phylotype in trickling filter biofilm and effluent, while a distinct “Nitrosomonas-like” lineage dominated in activated sludge. Prior time series indicated that this “Nitrosomonas-like” lineage was dominant when NO2 − levels in the trickling filter effluent (i.e., activated sludge influent) were low, while N. europaea became dominant in the activated sludge when NO2 − levels were high. This is consistent with the hypothesis that NO2 − production may cooccur with biofilm sloughing, releasing N. europaea from the trickling filter into the activated sludge bioreactor. Phylogenetic microarray (PhyloChip) analyses revealed significant spatial variation in taxonomic diversity, including a large excess of methanogens in the trickling filter relative to activated sludge and attenuation of Enterobacteriaceae across the transect, and demonstrated transport of a highly diverse microbial community via the trickling filter effluent to the activated sludge bioreactor. Our results provide compelling evidence that substantial immigration between coupled process units occurs and may exert significant influence over microbial community dynamics within staged bioreactors.





Similar content being viewed by others
References
Anthonisen AC, Loehr RC, Prakasam TBS, Srinath EG (1976) Inhibition of nitrification by ammonia and nitrous acid. J Water Pollut Con F 48(5):835–852
Brenner DJ, Farmer JJ III (2001) Family I. Enterobacteriaceae. In: Garrity GM, Brenner DJ, Krieg NR, Staley JT (eds) Bergey's manual of systematic bacteriology, vol 2, 2nd edn. Springer, New York
Brodie EL, DeSantis TZ, Parker JPM, Zubietta IX, Piceno YM, Andersen GL (2007) Urban aerosols harbor diverse and dynamic bacterial populations. Proc Natl Acad Sci U S A 104(1):299–304
Cottenie K (2005) Integrating environmental and spatial processes in ecological community dynamics. Ecol Lett 8(11):1175–1182
Curtis TP, Head IM, Graham DW (2003) Theoretical ecology for engineering biology. Environ Sci Technol 37(3):64A–70A
Elenter D, Milferstedt K, Zhang W, Hausner M, Morgenroth E (2007) Influence of detachment on substrate removal and microbial ecology in a heterotrophic/autotrophic biofilm. Water Res 41(20):4657–4671
Gray ND, Miskin IP, Kornilova O, Curtis TP, Head IM (2002) Occurrence and activity of archaea in aerated activated sludge wastewater treatment plants. Environ Microbiol 4(3):158–168
Jarrell KF (1985) Extreme oxygen sensitivity in methanogenic archaebacteria. Bioscience 35(5):298–302
Jones SE, McMahon KD (2009) Species-sorting may explain an apparent minimal effect of immigration on freshwater bacterial community dynamics. Environ Microbiol 11(4):905–913
Jones SE, Newton RJ, McMahon KD (2008) Potential for atmospheric deposition of bacteria to influence bacterioplankton communities. FEMS Microbiol Ecol 64(3):388–394
Kendall MM, Boone DR (2006) The order Methanosarcinales. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes: a handbook on the biology of bacteria, vol 3, 3rd edn. Springer Science + Business Media, LLC, pp 244–256
Kissel JC, McCarty PL, Street RL (1984) Numerical simulation of mixed-culture biofilm. J Environ Eng 110(2):393–411
Koops HP, Pommerening-Roser A (2001) Distribution and ecophysiology of the nitrifying bacteria emphasizing cultured species. FEMS Microbiol Ecol 37(1):1–9
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7(7):601–613
Lindström ES, Bergström A-K (2004) Influence of inlet bacteria on bacterioplankton assemblage composition in lakes of different hydraulic retention time. Limnol Oceanogr 49(1):125–136
Lindström ES, Forslund M, Algesten G, Bergström A-K (2006) External control of bacterial community structure in lakes. Limnol Oceanogr 51(1):339–342
Morgenroth E (2003) Detachment: an often overlooked phenomenon in biofilm research and modeling. In: Wuertz S, Wilderer PA, Bishop PL (eds) Biofilms in Wastewater Treatment: An Interdisciplinary Approach. IWA Publishing, London, pp 264–290
Morgenroth E, Wilderer PA (2000) Influence of detachment mechanisms on competition in biofilms. Water Res 34(2):417–426
Ofiteru ID, Lunn M, Curtis TP, Wells GF, Criddle CS, Francis CA, Sloan WT (2010) Combined niche and neutral effects in a microbial wastewater treatment community. Proc Natl Acad Sci U S A 107(35):15345–15350
Okabe S, Hirata K, Watanabe Y (1995) Dynamic changes in spatial microbial distribution in mixed-population biofilms: experimental results and model simulation. Water Sci Technol 32(8):67–74
Park HD, Noguera DR (2007) Characterization of two ammonia-oxidizing bacteria isolated from reactors operated with low dissolved oxygen concentrations. J Appl Microbiol 102(5):1401–1417
Rittmann BE, Laspidou CS (2003) Biofilm detachment. In: Bitton G (ed) Encyclopedia of environmental microbiology. Wiley, New York
Rittmann BE, McCarty PL (2001) Environmental biotechnology: principles and applications. McGraw-Hill Higher Education, New York
Shannon KE, Lee DY, Trevors JT, Beaudette LA (2007) Application of real-time quantitative PCR for the detection of selected bacterial pathogens during municipal wastewater treatment. Sci Total Environ 382(1):121–129
Shmida A, Wilson MV (1985) Biological determinants of species diversity. J Biogeogr 12(1):1–20
Sloan WT, Lunn M, Woodcock S, Head IM, Nee S, Curtis TP (2006) Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ Microbiol 8(4):732–740
Stewart PS (1993) A model of biofilm detachment. Biotechnol Bioeng 41(1):111–117. doi:10.1002/bit.260410115
Tchobanoglous G, Burton FL, Stensel HD (2002) Wastewater engineering: treatment and reuse. McGraw-Hill, New York
Tibshirani R, Hastie T, Narasimhan B, Chu G (2002) Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A 99(10):6567–6572
Wanner O, Gujer W (1986) A multispecies biofilm model. Biotechnol Bioeng 28(3):314–328
Wells GF (2011) Reexamining the engineered nitrogen cycle: microbial diversity, community dynamics, and immigration in nitrifying activated sludge bioreactors. Ph.D. Thesis. Stanford University: Stanford, CA, USA
Wells GF, Park H-D, Yeung C-H, Eggleston B, Francis CA, Criddle CS (2009) Ammonia-oxidizing communities in a highly aerated full-scale activated sludge bioreactor: betaproteobacterial dynamics and low relative abundance of Crenarchaea. Environ Microbiol 11(9):2310–2328
Wells GF, Park H-D, Eggleston B, Francis CA, Criddle CS (2011) Fine-scale bacterial community dynamics and the taxa-time relationship within a full-scale activated sludge bioreactor. Water Res 45(17):5476–5488
Wéry N, Lhoutellier C, Ducray F, Delgenès J-P, Godon J-J (2008) Behaviour of pathogenic and indicator bacteria during urban wastewater treatment and sludge composting, as revealed by quantitative PCR. Water Res 42(1–2):53–62
Wittebolle L, Boon N, Vanparys B, Heylen K, De Vos P, Verstraete W (2005) Failure of the ammonia oxidation process in two pharmaceutical wastewater treatment plants is linked to shifts in the bacterial communities. J Appl Microbiol 99(5):997–1006
Acknowledgments
We thank the PARWQCP staff for assisting with transect sampling and operational data monitoring. G.F.W. was supported by EPA STAR and NSF Graduate Research Fellowships. This work was funded by the Woods Institute for the Environment at Stanford University, by the PARWQCP, and by the Office of Biological and Environmental Research of the US DOE under contract no. DE-AC02-05CH11231 to Lawrence Berkeley National Laboratory.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 530 kb)
Rights and permissions
About this article
Cite this article
Wells, G.F., Wu, C.H., Piceno, Y.M. et al. Microbial biogeography across a full-scale wastewater treatment plant transect: evidence for immigration between coupled processes. Appl Microbiol Biotechnol 98, 4723–4736 (2014). https://doi.org/10.1007/s00253-014-5564-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5564-3