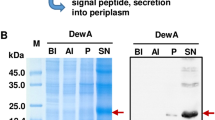
Abstract
The chaplin proteins are instrumental in the formation of reproductive aerial structures by the filamentous bacterium Streptomyces coelicolor. They lower the water surface tension thereby enabling aerial growth. In addition, chaplins provide surface hydrophobicity to the aerial hyphae by assembling on the cell surface into an amphipathic layer of amyloid fibrils. We here show that mixtures of cell wall-extracted chaplins can be used to modify a variety of hydrophilic and hydrophobic surfaces in vitro thereby changing their nature. Assembly on glass leads to a protein coating that makes the surface hydrophobic. Conversely, the assembly of chaplins on hydrophobic surfaces renders them hydrophilic. Furthermore, we show that chaplins can stabilize emulsions of oil into water and have an unprecedented surface activity at high pH. Interestingly, this high surface activity coincides with the interfacial assembly of chaplins into a semi-liquid membrane, as opposed to the rigid membrane formed at neutral pH. This semi-liquid membrane possibly represents a trapped intermediate in the assembly process towards the more rigid amyloidal conformation. Taken together, our data shows that chaplins are suitable candidate proteins for a wide range of biotechnological applications.







Similar content being viewed by others
References
Bilewicz R, Witomski J, Van der Heyden A, Tagu D, Palin B, Rogalska E (2004) Modification of electrodes with self-assembled hydrophobin layers. J Phys Chem B 105(40):9772–9777
Blanco LP, Evans ML, Smith DR, Badtke MP, Chapman MR (2012) Diversity, biogenesis, and function of microbial amyloids. Trends Microbiol 20(2):66–73
Bokhove M, Claessen D, de Jong W, Dijkhuizen L, Boekema EJ, Oostergetel GT (2013) Chaplins of Streptomyces coelicolor self-assemble into two distinct functional amyloids. J Struct Biol. doi:10.1016/j.jsb.2013.08.013, in press
Capstick DS, Jomaa A, Hanke C, Ortega J, Elliot MA (2011) Dual amyloid domains promote differential functioning of the chaplin proteins during Streptomyces aerial morphogenesis. Proc Natl Acad Sci U S A 108(24):9821–9826
Chater KF, Horinouchi S (2003) Signaling early developmental events in two highly diverged Streptomyces species. Mol Microbiol 48(1):9–15
Claessen D, Wösten HAB, van Keulen G, Faber OG, Alves AM, Meijer WG, Dijkhuizen L (2002) Two novel homologous proteins of Streptomyces coelicolor and Streptomyces lividans are involved in the formation of the rodlet layer and mediate attachment to a hydrophobic surface. Mol Microbiol 44(6):1483–1492
Claessen D, Rink R, de Jong W, Siebring J, de Vreugd P, Boersma FG, Dijkhuizen L, Wösten HAB (2003) A novel class of secreted hydrophobic proteins in involved in aerial hyphae formation in Streptomyces coelicolor by forming amyloid-like fibrils. Genes Dev 17(14):1714–1726
Claessen D, Stokroos I, Deelstra HJ, Penninga NA, Bormann C, Salas JA, Dijkhuizen L, Wösten HAB (2004) The formation of the rodlet layer of Streptomycetes is the result of the interplay between rodlins and chaplins. Mol Microbiol 53(2):433–443
Claessen D, de Jong W, Dijkhuizen L, Wösten HAB (2006) Regulation of Streptomyces development: reach for the sky! Trends Microbiol 14(7):313–319
de Jong W, Wösten HAB, Dijkhuizen L, Claessen D (2009) Attachment of Streptomyces coelicolor is mediated by amyloidal fimbriae that are anchored to the cell surface via cellulose. Mol Microbiol 73(6):1128–1140
de Jong W, Vijgenboom E, Dijkhuizen L, Wösten HAB, Claessen D (2012) SapB and the rodlins are required for development of Streptomyces coelicolor in high osmolarity media. FEMS Microbiol Lett 329(2):154–159
Duong A, Capstick DS, Di Berardo C, Findlay KC, Hesketh A, Hong HJ, Elliot MA (2012) Aerial development in Streptomyces coelicolor requires sortase activity. Mol Microbiol 83(5):992–1005
Eisenberg D, Jucker M (2012) The amyloid state of proteins in human diseases. Cell 148(6):1188–1203
Elliot MA, Talbot NJ (2004) Building filaments in the air: aerial morphogenesis in bacteria and fungi. Curr Opin Microbiol 7(6):594–601
Elliot MA, Karoonuthaisiri N, Huang J, Bibb MJ, Cohen SN, Kao CM, Buttner MJ (2003) The chaplins: a family of hydrophobic cell-surface proteins involved in aerial mycelium formation in Streptomyces coelicolor. Genes Dev 17(14):1727–1740
Falabella P, Riviello L, Pascale M, Lelio ID, Tettamanti G, Grimaldi A, Iannone C, Monti M, Pucci P, Tamburro AM, Deeguileor M, Gigliotti S, Pennacchio F (2012) Functional amyloids in insect immune response. Insect Biochem Mol Biol 42(3):203–211
Flärdh K, Buttner MJ (2009) Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nat Rev Microbiol 7(1):36–49
Fodera V, Groenning M, Vetri V, Librizzi F, Spagnolo S, Cornett C, Olsen L, van der Weert M, Leone M (2008) Thioflavin T hydroxylation at basic pH and its effect on amyloid fibril detection. J Phys Chem 112:15174–15181
Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE, Kelly JW (2006) Functional amyloid formation within mammalian tissue. PLOS Biol 4(1):e6
Gras SL, Claessen D (2013) Functional amyloid fibrils: lessons from microbes. In: Havlicek V, Spizek J (eds) Natural Products Analysis: instrumentation, Methods, and Applications. Wiley (in press)
Gebbink MFBH, Claessen D, Bouma B, Dijkhuizen L, Wösten HAB (2005) Amyloids—a functional coat for microorganisms. Nat Rev Microbiol 3(4):333–341
Hozzein WN, Ali MIA, Hammouda O, Mousa AS, Goodfellow M (2011) Streptomyces sannurensis sp. nov., a new alkaliphilic member of the genus Streptomyces isolated from Wadi Sannur in Egypt. Afr J Microbiol Res 5(11):1329–1334
Janssen MI, van Leeuwen MB, van Kooten TG, de Vries J, Dijkhuizen L, Wösten HAB (2004) Promotion of fibroblast activity by coating with hydrophobins in the β-sheet end state. Biomaterials 25(14):2731–2739
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Li WJ, Zhang YG, Zhang YQ, Tang SK, Xu P, Xu LH, Jiang CL (2005) Streptomyces sodiiphilus sp. Nov., a novel alkaliphilic actinomycete. Int J Syst Evol Microbiol 55(3):1329–1333
Linder M, Szilvay GR, Nakari-Setala T, Soderlund H, Penttila M (2002) Surface adhesion of fusion proteins containing the hydrophobins HFBI and HFBII from Trichoderma reesei. Protein Sci 11(9):2257–2266
Linder MB, Szilvay GR, Nakari-Setala T, Penttila ME (2005) Hydrophobins: the protein-amphiphiles of filamentous fungi. FEMS Microbiol Rev 29(5):877–896
Sawyer EB, Claessen D, Haas M, Hurgobin B, Gras SL (2011) The assembly of individual chaplin peptides from Streptomyces coelicolor into functional amyloid fibrils. PLoS One 6(4):18839–18839
Scholtemeijer K, Janssen MI, van Leeuwen MB, van Kooten TG, Hektor H, Wösten HAB (2004) The use of hydrophobins to functionalize surfaces. Bio-Med Mater Eng 14(4):447–454
Scholtmeijer K, de Vocht ML, Rink R, Robillard GT, Wösten HAB (2009) Assembly of the fungal SC3 hydrophobin into functional amyloid fibrils depends on its concentration and is promoted by cell wall polysaccharides. J Biol Chem 284(39):26309–26314
Srinivasan R, Jones EM, Liu K, Ghiso J, Marchant RE, Zagorski MG (2003) pH-dependent amyloid and protofibril formation by the ABri peptide of familial British dementia. J Mol Biol 333(5):1003–1023
Stauffer CE (1965) The measurement of surface tension by the pendant drop technique. J Phys Chem 69(6):1933–1938
Sunde M, Kwan AHY, Templeton MD, Beever RE, Mackay JP (2008) Structural analysis of hydrophobins. Micron 39(7):773–784
Wösten HAB (2001) Hydrophobins: multipurpose proteins. Annu Rev Microbiol 55:625–646
Xu W, Zhang C, Derreumaux P, Graslund A, Morozova-Roche L, Mu Y (2011) Intrinsic determinants of Abeta12-24 pH-dependent self-assembly revealed by combined computational and experimental studies. PLoS One 6(9):1–12
Acknowledgments
We would like to thank Karin Scholtmeijer and Han Wösten for stimulating discussions and their help with the water contact angle and surface tension measurements.
Author information
Authors and Affiliations
Corresponding authors
Additional information
David Matthias Ekkers and Dennis Claessen contributed equally to this manuscript.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ekkers, D.M., Claessen, D., Galli, F. et al. Surface modification using interfacial assembly of the Streptomyces chaplin proteins. Appl Microbiol Biotechnol 98, 4491–4501 (2014). https://doi.org/10.1007/s00253-013-5463-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5463-z