Abstract
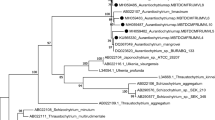
Thraustochytrids are ubiquitous marine osmo-heterotrophic fungi-like microorganisms with only about 40 identified species till now. In this study, a total of 60 thraustochytrid strains were isolated from marine coastal habitats. Analysis of 18S rRNA gene sequences revealed that they belonged to three genera, i.e., Schizochytrium, Aurantiochytrium, and Thraustochytrium. All of the isolates were found to show considerable cellulolytic and lipolytic activities. Strains of Aurantiochytrium sp. and Thraustochytrium sp. were found to produce the highest levels of extracellular polysaccharides (EPS), which reached 345 μg ml−1 in the growth media. Fourier transform infrared (FTIR) spectra of the EPS samples derived from two thraustochytrids (PKU#Sed1 and #SW1) displayed peaks for carbohydrates, proteins, lipids, uronic acids, and nucleic acids. Fatty acid profiles of four thraustochytrids comprised of palmitic acid (C16:0) and docosahexaenoic acid (DHA) as their major constituents. Schizochytrium sp. demonstrated the highest DHA production at 44 % of total fatty acids (TFA) with biomass and DHA yield of 7.1 and 1.6 g l−1, respectively, on the fourth day of growth. All the four isolates exhibited considerable production of palmitic acid (16:0) in their fatty acid profiles ranging from 35 to 50 % TFA. This is the first report on extracellular enzymes, EPS, and DHA production from thraustochytrids isolated from the coastal habitats of China.







Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Arafiles K, Alcantara J, Batoon J, Galura F, Cordero P, Leaño E, Dedeles G (2011) Cultural optimization of thraustochytrids for biomass and fatty acid production. Mycosphere 2:521–531
Bajpai P, Bajpai PK, Ward OP (1991) Production of docosahexaenoic acid by Thraustochytrium aureum. Appl Microbiol Biotechnol 35:706–710
Benner R, Moran MA, Hodson RE (1986) Biogeochemical cycling of lignocellulosic carbon in marine and freshwater ecosystems: relative contributions of prokaryotes and eukaryotes. Limnol Oceanogr 31:89–100
Bhosle NB, Garg A, Sawant SS, Wagh A (1995) Isolation and partial chemical analysis of exopolysaccharides from the marine fouling diatom Navicula subinflata. Bot Mar 38:103–110
BoekeMa B, Beselin A, Breuer M, Hauer B, Koster M, Rosenau F, Jaeger KE, Tommassen J (2007) Hexadecane and Tween 80 stimulate lipase production in Burkholdetia glumae by different mechanisms. Appl Environ Microbiol 73(12):3838–3844
Bongiorni L, Pignataro L, Santangelo G (2004) Thraustochytrids (fungoid protists): an unexplored component of marine sediment microbiota. Sci Mar 68:43–48
Bongiorni L, Jain R, Raghukumar S, Aggarwal RK (2005a) Thraustochytrium gaertnerium sp. nov.: a new thraustochytrid stramenopilan protist from mangroves of Goa, India. Protist 156(3):303–315
Bongiorni L, Pusceddu A, Danovaro R (2005b) Enzymatic activities of epiphytic and benthic thraustochytrids involved in organic matter degradation. Aquat Microb Ecol 41:299–305
Bramono K, Yarnazaki M, Tsuboi R, Ogawa H (2006) Comparison of proteinase, lipase and alpha-glucosidase activities from the clinical isolates of Candida species. Jpn J Infect Dis 59(2):73–76
Bremer GB (1976) The ecology of marine lower fungi. In: Jones EBG (ed) Recent advances in aquatic mycology. Elek Science, London, pp 313–333
Bremer GB, Talbot G (1995) Cellulolytic enzyme-activity in the marine protist Schizochytrium aggregatum. Bot Mar 38(1):37–41
Carder JH (1986) Detection and quantitation of cellulase by congo red staining of substrates in a cup-plate diffusion assay. Anal Biochem 153(1):75–79
Cavalier-Smith T, Allsopp M, Chao EE (1994) Thraustochytrids are chromists, not fungi: 18S rRNA signatures of Heterokonta. Philos Trans R Soc Lond Ser B Biol Sci 346(1318):387–397
Damare V, Raghukumar S (2006) Morphology and physiology of the marine straminipilan fungi, the aplanochytrids isolated from the equatorial Indian Ocean. Indian J Mar Sci 35(4):326–340
Damare S, Raghukumar C, Muraleedharan UD, Raghukumar S (2006) Deep-sea fungi as a source of alkaline and cold-tolerant proteases. Enzyme Microb Technol 39(2):172–181
Decho AW (1990) Microbial exopolymer secretions in ocean environment: their role(s) in food webs and marine processes. Oceanogr Mar Biol Annu Rev 28:73–153
Decho AW (1993) Methods for the observation and use in feeding experiments of microbial exopolymer. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis, Chelsea, pp 685–694
Dighton J (2003) The role of fungi in ecosystem processes. Marcel Dekker, New York
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for the determination of sugars and related substances. Anal Chem 28:350–356
Eriksson KE, Hamp SG (1978) Regulation of Endo-1,4-β-glucanase production in Sporotrichum pulverulentum. Eur J Biochem 90(1):183–190
Fan KW, Vrijmoed LLP, Jones EBG (2002) Physiological studies of subtropical mangrove thraustochytrids. Bot Mar 45(1):50–57
Fisher L, Nicholls D, Sanderson K (2008) Production of biodiesel. World Intellect Prop Organ 067605
Flemming H, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8:623–633
Gaertner A (1968) Eine Methode des quantitativen Nachweises niederer mit Pollen koederbarer Pilze im Meerwasser und im Sediment. Verӧff Inst Meeresforsch Bremerh 3:75–92
Greenspan P, Mayer EP, Fowler SD (1985) Nile Red: a selective fluorescent stain for intracellular lipid droplets. J. Cell Biol 100:965–973
Gupta N, Rathi P, Gupta R (2002) Simplified para-nitrophenyl palmitate assay for lipases and esterases. Anal Biochem 311(1):98–99
Gutierrez A, Martinez AT, Prieto A (1996) Structural characterization of extracellular polysaccharides produced by fungi from the genus Pleurotus. Carbohydr Res 281:143–154
Honda D, Yokochi T, Nakahara T, Raghukumar S, Nakagiri A, Schaumann K, Higashihara T (1999) Molecular phylogeny of labyrinthulids and thraustochytrids based on the sequencing of 18S ribosomal RNA gene. J Eukaryot Microbiol 46(6):637–647
Huang JZ, Aki T, Yokochi T, Nakahara T, Honda D, Kawamoto S, Shigeta S, Ono K, Suzuki O (2003) Grouping newly isolated docosahexaenoic acid-producing thraustochytrids based on their polyunsaturated fatty acid profiles and comparative analysis of 18S rRNA genes. Mar Biotechnol 5(5):450–457
Jaeger KE, Reetz MT (1998) Microbial lipases form versatile tools for biotechnology. Trends Biotechnol 16(9):396–403
Jain R, Raghukumar S, Tharanathan R, Bhosle NB (2005) Extracellular polysaccharide production by thraustochytrid protists. Mar Biotechnol 7:184–192
Jain R, Raghukumar S, Sambaiah K, Kumon Y, Nakahara T (2007) Docosahexaenoic acid accumulation in thraustochytrids: search for the rationale. Mar Biol 151(5):1657–1664
Jakobsen AN, Aasen IM, Josefsen KD, Strøm AR (2008) Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain T66: effects of N and P starvation and O2 limitation. Appl Microbiol Biotechnol 80:297–306
Kamlangdee N, Fan KW (2003) Polyunsaturated fatty acids production by Schizochytrium sp. isolated from mangrove. J Sci Technol 25:643–650
Kanchana R, Muraleedharan UD, Raghukumar S (2011) Alkaline lipase activity from the marine protists, thraustochytrids. World J Microb Biotechnol 27(9):2125–2131
Kinsella JE (1987) Seafoods and fish oils in human health and disease. Marcel Dekker, New York
Knothe G (2007) Some aspects of biodiesel oxidative stability. Fuel Process Technol 88:669–677
Leander CA, Porter D, Leander BS (2004) Comparative morphology and molecular phylogeny of aplanochytrids (Labyrinthulomycota). Eur J Protistol 40(4):317–328
Lee Chang KJ, Dunstan GA, Abell GC, Clementson LA, Blackburn SI, Nichols PD, Koutoulis A (2012) Biodiscovery of new Australian thraustochytrids for production of biodiesel and long-chain omega-3 oils. Appl Microbiol Biotechnol 93:2215–2231
Lewis TE, Nichols PD, McMeekin TA (1999) The biotechnological potential of thraustochytrids. Mar Biotechnol 1:580–587
Li ZY, Ward OP (1994) Production of docosahexaenoic acid by Thraustochytrium roseum. J Ind Microbiol 13:238–241
Lima VMG, Krieger N, Sarquis MIM, Mitchell DA, Ramos LP, Fontana JD (2003) Effect of nitrogen and carbon sources on lipase production by Penicillium aurantiogriseum. Food Technol Biotechnol 41(2):105–110
Mayer C, Moritz R, Kirschner C, Borchard W, Maibaum R, Wingender J, Flemming HC (1999) The role of intermolecular interactions: studies on model systems for bacterial biofilms. Int J Biol Macromol 26:3–16
Monyem A, Canakci M, Van Gerpen JH (2000) Investigation of biodiesel thermal stability under simulated in-use conditions. Appl Eng Agric 16:373–378
Myklestad SM, Haug A (1972) Production of carbohydrates by the marine diatom Chaetoceros affinis var. willei (Gran) Hustedt. II. Preliminary investigation of the extracellular polysaccharide. J Exp Mar Biol Ecol 9:137–144
Nagano N, Matsui S, Kuramura T, Taoka Y, Honda D, Hayashi M (2011) The distribution of extracellular cellulase activity in marine eukaryotes, thraustochytrids. Mar Biotechnol 13(2):133–136
Newell SY, Fell JW (1982) Mycoflora of turtlegrass (Thalassia testudinum Konig) as recorded after seawater incubation. Bot Mar 23:265–275
Pinsirodom P, Parkin KL (2001) Lipase assays. Current protocols in food analytical chemistry. Wiley, New York
Porter D (1990) Labyrinthulomycota. In: Margulis L, Corliss JO, Melkonian M, Chapman D (eds) Handbook of Protoctista. Jones and Bartlett, Boston, pp 388–398
Raghukumar S (2002) Ecology of the marine protists, the Labyrinthulomycetes (Thraustochytrids and Labyrinthulids). Eur J Protistol 38(2):127–145
Raghukumar S, Damare VS (2011) Increasing evidence for the important role of Labyrinthulomycetes in marine ecosystems. Bot Mar 54:3–11
Raghukumar S, Raghukumar C (1999) Thraustochytrid fungoid protists in faecal pellets of the tunicate Pegea confoederata, their tolerance to deep-sea conditions and implication in degradation processes. Mar Ecol Prog Ser 190:133–140
Raghukumar S, Sharma S, Raghukumar C, Sathe-Pathak V (1994) Thraustochytrid and fungal component of marine detritus. IV. Laboratory studies on decomposition of the leaves of the mangrove Rhizophora apiculata Blume. J Exp Mar Biol Ecol 183:113–131
Raghukumar S, Sathepathak V, Sharma S, Raghukumar C (1995) Thraustochytrid and fungal component of marine detritus. III. Field studies on decomposition of leaves of the mangrove Rhizophora apiculata. Aquat Microb Ecol 9(2):117–125
Raghukumar S, Anil AC, Khandeparkar L, Patil JS (2000) Thraustochytrid protists as a component of marine microbial films. Mar Biol 136:603–609
Rajan A, Kumar DRS, Nair AJ (2011) Isolation of a novel alkaline lipase producing fungus Aspergillus fumigatus MTCC 9657 from aged and crude rice bran oil and quantification by HPTLC. Int J Biol Chem 5:116–126
Sathe-pathak V, Raghukumar S, Raghukumar C, Sharma S (1993) Thraustochytrid and fungal component of marine detritus. I. Field studies on decomposition of the brown alga Sargassum cinereum. J Agric Ind J Mar Sci 22(3):159–167
Sharma S, Raghukumar C, Raghukumar S, Sathe-pathak V, Chandramohan D (1994) Thraustochytrid and fungal component of marine detritus II. Laboratory studies on decomposition of the brown alga Sargassum cinereum. J Agric J Exp Mar Biol Ecol 175(2):227–242
Sheng G, Yu H, Wang C (2006) FTIR-spectctral analysis of two photosynthetic H2-producing strains and their extracellular polymeric substances. Appl Microbiol Biotechnol 73:204–210
Simopoulos AP (1989) Summary of the NATO advanced research workshop on dietary omega 3 and omega 6 fatty acids: biological effects and nutritional essentiality. J Nutr 119:521–528
Singh A, Wilson S, Ward OP (1996) Docosahexaenoic acid (DHA) production by Thraustochytrium sp. ATCC 20892. World J Microbiol Biotechnol 12:76–81
Singh R, Gupta N, Goswami VK, Gupta R (2006) A simple activity staining protocol for lipases and esterases. Appl Microbiol Biotechnol 70(6):679–682
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases—production, applications and challenges. J Sci Ind Res 64(11):832–844
Sutherland IW (1985) Biosynthesis and composition of gram negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol 39:243–270
Sutherland IW (1998) Novel and established applications of microbial polysaccharides. Trends Biotechnol 16:41–46
Takahata K, Monobe KI, Tada M, Weber PC (1998) The benefits and risks of n-3 polyunsaturated fatty acids. Biosci Biotechnol Biochem 62:2079–2085
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596–1599
Taoka Y, Nagano N, Okita Y, Izumida H, Sugimoto S, Hayashi M (2009) Extracellular enzymes produced by marine eukaryotes, thraustochytrids. Biosci Biotechnol Biochem 73(1):180–182
Taoka Y, Nagano N, Okita Y, Izumida H, Sugimoto S, Hayashi M (2011) Effect of Tween 80 on the growth, lipid accumulation and fatty acid composition of Thraustochytrium aureum ATCC 34304. J Biosci Bioeng 111:420–424
Than PP, Del Castillo CS, Yoshikawa T, Sakata T (2004) Extracellular protease production of bacteriolytic bacteria isolated from marine environments. Fish Sci 70(4):659–666
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Tsui CKM, Marshall W, Yokoyama R, Honda D, Lippmeier JC, Craven KD, Peterson PD, Berbee ML (2009) Labyrinthulomycetes phylogeny and its implications for the evolutionary loss of chloroplasts and gain of ectoplasmic gliding. Mol Phylogenet Evol 50(1):129–140
Van de Peer Y, Baldaufrid SL, Doolittle WF, Meyerid A (2000) An updated and comprehensive rRNA phylogeny of (crown) eukaryotes based on rate-calibrated evolutionary distances. J Mol Evol 51(6):565–576
Volkman JK, Jeffrey SW, Nichols PD, Rogers GI, Garland CD (1989) Fatty acid and lipid composition of 10 species of microalgae used in mariculture. J Exp Mar Biol Ecol 128:219–240
Whitfield C (1988) Bacterial extracellular polysaccharides. Can J Microbiol 34:415–420
Wilkens SL, Maas EW (2012) Development of a novel technique for axenic isolation and culture of thraustochytrids from New Zealand marine environments. J Appl Microbiol 112(2):346–352
Williams AG, Wimpenny JWT (1977) Exopolysaccharide production by Pseudomonas NCIB 11264 grown in batch culture. J Gen Microbiol 102:13–21
Williams AG, Wimpenny JWT (1978) Exopolysaccharide production by Pseudomonas NCIB 11264 grown in continuous culture. J Gen Microbiol 104:47–57
Wingender J, Strathmann M, Rode A, Leis A, Flemming HC (2001) Isolation and biochemical characterization of extracellular polymeric substances from Pseudomonas aeruginosa. Methods Enzymol 336:302–314
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T (1997) Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J Am Oil Chem Soc 74:1431–1434
Yang HL, Lu CK, Chen SF, Chen YM (2010) Isolation and characterization of Taiwanese heterotrophic microalgae: screening of strains for docosahexaenoic acid (DHA) production. Mar Biotechnol 12(2):173–185
Yokochi T, Honda D, Higashihara T, Nakahara T (1998) Optimization of docosahexaenoic acid production by Schizochytrium limacinum SR21. Appl Microbiol Biotechnol 49:72–76
Acknowledgments
This work was partially funded by National Science Foundation of China grant 31170109 (GYW) and Shenzhen Development and Reform Commission grant 835 (GYW). Members of Shenzhen Key Lab of Nano-Micro Material Research, Peking University, are acknowledged for their technical support during FTIR analysis of EPS samples. All the authors are thankful to the Shenzhen Marine environment and resource monitoring center for the facilities extended during sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Liu and P. Singh contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 233 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Singh, P., Sun, Y. et al. Culturable diversity and biochemical features of thraustochytrids from coastal waters of Southern China. Appl Microbiol Biotechnol 98, 3241–3255 (2014). https://doi.org/10.1007/s00253-013-5391-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5391-y