Abstract
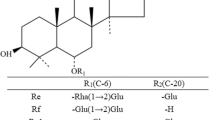
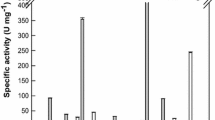
The hydrolytic activity of a recombinant β-glycosidase from Dictyoglomus turgidum that specifically hydrolyzed the xylose at the C-6 position and the glucose in protopanaxatriol (PPT)-type ginsenosides followed the order Rf > Rg1 > Re > R1 > Rh1 > R2. The production of aglycone protopanaxatriol (APPT) from ginsenoside Rf was optimal at pH 6.0, 80 °C, 1 mg ml−1 Rf, and 10.6 U ml−1 enzyme. Under these conditions, D. turgidum β-glycosidase converted ginsenoside R1 to APPT with a molar conversion yield of 75.6 % and a productivity of 15 mg l−1 h−1 after 24 h by the transformation pathway of R1 → R2 → Rh1 → APPT, whereas the complete conversion of ginsenosides Rf and Rg1 to APPT was achieved with a productivity of 1,515 mg l−1 h−1 after 6.6 h by the pathways of Rf → Rh1 → APPT and Rg1 → Rh1 → APPT, respectively. In addition, D. turgidum β-glycosidase produced 0.54 mg ml−1 APPT from 2.29 mg ml−1 PPT-type ginsenosides of Panax ginseng root extract after 24 h, with a molar conversion yield of 43.2 % and a productivity of 23 mg l−1 h−1, and 0.62 mg ml−1 APPT from 1.35 mg ml−1 PPT-type ginsenosides of Panax notoginseng root extract after 20 h, with a molar conversion yield of 81.2 % and a productivity of 31 mg l−1 h−1. This is the first report on the APPT production from ginseng root extract. Moreover, the concentrations, yields, and productivities of APPT achieved in the present study are the highest reported to date.






Similar content being viewed by others
References
Attele AS, Wu JA, Yuan CS (1999) Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol 58:1685–1693
Bae EH, Shin JE, Kim DH (2005) Metabolism of ginsenoside Re by human intestinal microflora and its estrogenic effect. Bio Pharm Bull 28:1903–1908
Chen Y, Xu YJ, Zhu Y, Li XL (2013) Anti-cancer effects of ginsenoside compound K on pediatric acute myeloid leukemia cells. Cancer Cell Int 13:24
Chi H, Ji GE (2005) Transformation of ginsenosides Rb1 and Re from Panax ginseng by food microorganisms. Biotechnol Lett 27:765–771
Choo MK, Park EK, Han MJ, Kim DH (2003) Antiallergic activity of ginseng and its ginsenosides. Planta Med 69:518–522
Cui CH, Kim SC, Im WT (2013) Characterization of the ginsenoside transforming recombinant β-glucosidase from Actinosynnema mirum and bioconversion of major ginsenosides into minor ginsenosides. Appl Microbiol Biotechnol 97:649–659
Han Y, Sun B, Hu X, Zhang H, Jiang B, Spranger MI, Zhao Y (2007) Transformation of bioactive compounds by Fusarium sacchari fungus isolated from the soil cultivated ginseng. J Agric Food Chem 55:9373–9379
Hasegawa H, Suzuki R, Nagaoka T, Tezuka Y, Kadota S, Saiki I (2002) Prevention of growth and metastasis of murine melanoma through enhanced natural-killer cytotoxicity by fatty acid conjugate of protopanaxatriol. Bio Pharm Bull 25:861–866
Huang CR, Wang GJ, Li H, Xie HT, Sun HG, Lv H, Lv T (2006) Sensitive and selective liquid chromatography electrospray ionisation mass spectrometry analysis of astragaloside-IV in rat plasma. J Pharm Biomed Anal 40:788–793
Kim MK, Lee JW, Lee KY, Yang DC (2005) Microbial conversion of major ginsenoside Rb1 to pharmaceutically active minor ginsenoside Rd. J Microbiol 43:456–462
Kim BH, Lee SY, Cho HJ, You SN, Kim YJ, Park YM, Lee JK, Baik MY, Park CS, Ahn SC (2006) Biotransformation of Korean Panax ginseng by Pectinex. Biol Pharm Bull 29:2472–2478
Kim JK, Cui CH, Yoon MH, Kim SC, Im WT (2012) Bioconversion of major ginsenosides Rg1 to minor ginsenoside F1 using novel recombinant ginsenoside hydrolyzing glycosidase cloned from Sanguibacter keddieii and enzyme characterization. J Biotechnol 161:294–301
Ko SR, Choi KJ, Suzuki K, Suzuki Y (2003) Enzymatic preparation of ginsenosides Rg2, Rh1, and F1. Chem Pharm Bull 51:404–408
Lee GW, Kim KR, Oh DK (2012) Production of rare ginsenosides (compound Mc, compound Y and aglycon protopanaxadiol) by β-glucosidase from Dictyoglomus turgidum that hydrolyzes beta-linked, but not α-linked, sugars in ginsenosides. Biotechnol Lett 34:1679–1686
Lee GW, Yoo MH, Shin KC, Kim KR, Kim YS, Lee KW, Oh DK (2013) β-Glucosidase from Penicillium aculeatum hydrolyzes exo-, 3-O-, and 6-O-β-glucosides but not 20-O-β-glucoside and other glycosides of ginsenosides. Appl Microbiol Biotechnol 97:6315–6324
Li BH, Wang CZ, He TC, Yuan CS, Du W (2010) Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett 289:62–70
Liu L, Gu LJ, Zhang DL, Wang Z, Wang CY, Li Z, Sung CK (2010) Microbial conversion of rare ginsenoside Rf to 20(S)-protopanaxatriol by Aspergillus niger. Biosci Biotechnol Biochem 74:96–100
Ni HX, Yu NJ, Yang XH (2010) The study of ginsenoside on PPARgamma expression of mononuclear macrophage in type 2 diabetes. Mol Biol Rep 37:2975–2979
Noh KH, Oh DK (2009) Production of the rare ginsenosides compound K, compound Y, and compound Mc by a thermostable β-glycosidase from Sulfolobus acidocaldarius. Bio Pharm Bull 32:1830–1835
Noh KH, Son JW, Kim HJ, Oh DK (2009) Ginsenoside compound K production from ginseng root extract by a thermostable β-glycosidase from Sulfolobus solfataricus. Biosci Biotechnol Biochem 73:316–321
Park CS, Yoo MH, Noh KH, Oh DK (2010) Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotechnol 87:9–19
Popovich DG, Kitts DD (2002) Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys 406:1–8
Wang Y, Liu TH, Wang W, Wang BX (2001) Research on the transformation of ginsenoside Rg1 by intestinal flora. China J Chin Mater Med 26:188–190
Wang YZ, Chen J, Chu SF, Wang YS, Wang XY, Chen NH, Zhang JT (2009) Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1's metabolites ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci 109:504–510
Wang DM, Yu HS, Song JG, Xu YF, Jin FX (2012) Enzyme kinetics of ginsenosidase type IV hydrolyzing 6-O-multi-glycosides of protopanaxatriol type ginsenosides. Process Biochem 47:133–138
Wei Y, Zhao W, Zhang Q, Zhao Y, Zhang Y (2011) Purification and characterization of a novel and unique ginsenoside Rg1-hydrolyzing β-D-glucosidase from Penicillium sclerotiorum. Acta Biochim Biophys Sin 43:226–231
Yoo MH, Yeom SJ, Park CS, Lee KW, Oh DK (2011) Production of aglycon protopanaxadiol via compound K by a thermostable β-glycosidase from Pyrococcus furiosus. Appl Microbiol Biotechnol 89:1019–1028
Yu HS, Gong J, Zhang C, Jin FX (2002) Purification and characterization of ginsenoside-β-L-rhamnosidase. Chem Pharm Bull 50:175–178
Zhang Y, Takashina K, Saito H, Nishiyama N (1994) Anti-aging effect of DX-9386 in senescence accelerated mouse. Biol Pharm Bull 17:866–868
Zhou W, Yan Q, Li JY, Zhang XC, Zhou P (2008) Biotransformation of Panax notoginseng saponins into ginsenoside compound K production by Paecilomyces bainier sp 229. J Appl Microbiol 104:699–706
Acknowledgments
This work was supported by the Basic Research Lab program (no. 2010–0019306), the National Research Foundation, the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, HJ., Shin, KC., Lee, GW. et al. Production of aglycone protopanaxatriol from ginseng root extract using Dictyoglomus turgidum β-glycosidase that specifically hydrolyzes the xylose at the C-6 position and the glucose in protopanaxatriol-type ginsenosides. Appl Microbiol Biotechnol 98, 3659–3667 (2014). https://doi.org/10.1007/s00253-013-5302-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5302-2