Abstract
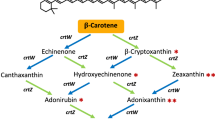
Escherichia coli cells that express the full six carotenoid biosynthesis genes (crtE, crtB, crtI, crtY, crtZ, and crtX) of the bacterium Pantoea ananatis have been shown to biosynthesize zeaxanthin 3,3′-β-d-diglucoside. We found that this recombinant E. coli also produced a novel carotenoid glycoside that contained a rare carbohydrate moiety, quinovose (chinovose; 6-deoxy-d-glucose), which was identified as 3-β-glucosyl-3′-β-quinovosyl zeaxanthin by chromatographic and spectroscopic analyses. The chirality of the aglycone of these zeaxanthin glycosides had been shown to be 3R,3′R, in which the hydroxyl groups were formed with the CrtZ enzyme. It was here demonstrated that zeaxanthin synthesized from β-carotene with CrtR or CYP175A1, the other hydroxylase with similar catalytic function to CrtZ, possessed the same stereochemistry. It was also suggested that the singlet oxygen-quenching activity of zeaxanthin 3,3′-β-d-diglucoside, which has a chemical structure close to the new carotenoid glycoside, was superior to that of zeaxanthin.


Similar content being viewed by others
References
Blasco F, Kauffmann I, Schmid RD (2004) CYP175A1 from Thermus thermophilus HB27, the first β-carotene hydroxylase of the P450 superfamily. Appl Micrbiol Biotechnol 64:671–674
Britton G, Liaaen-Jensen S, Pfander H (2004) Carotenoids handbook. Birkhäuser, Basel
Choi SK, Matsuda S, Hoshino T, Peng X, Misawa N (2006) Characterization of bacterial β-carotene 3,3′-hydroxylases, CrtZ, and P450 in astaxanthin biosynthetic pathway and adonirubin production by gene combination in Escherichia coli. Appl Microbiol Biotechnol 72:1238–1246
Hirayama O, Nakamura K, Hamada S, Kobayashi Y (1994) Singlet oxygen quenching ability of naturally occurring carotenoids. Lipids 29:149–150
Hundle BS, O'Brien DA, Alberti M, Beyer P, Hearst JE (1992) Functional expression of zeaxanthin glucosyltransferase from Erwinia herbicola and a proposed uridine diphosphate binding site. Proc Natl Acad Sci U S A 89:9321–9325
Iwamoto T, Hosoda K, Hirano R, Kurata H, Matsumoto A, Miki W, Kamiyama M, Itakura H, Yamamoto S, Kondo K (2000) Inhibition of low-density lipoprotein oxidation by astaxanthin. J Atheroscler Thromb 7:216–222
Kobayashi M, Sakamoto Y (1999) Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol Lett 21:265–269
Krinsky NI, Landrum JT, Bone RA (2003) Biological mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr 23:171–201
Makino T, Harada H, Ikenaga H, Matsuda S, Takaichi S, Shindo K, Sandmann G, Ogata T, Misawa N (2008) Characterization of cyanobacterial carotenoid ketolase CrtW and hydroxylase CrtR by complementation analysis in Escherichia coli. Plant Cell Physiol 49:1867–1878
Maoka T, Arai A, Shimizu M, Matsuno T (1986) The first isolation of enantiomeric and meso-zeaxanthin in nature. Comp Biochem Physiol 83B:121–124
Masamoto K, Misawa N, Kaneko T, Kikuno R, Toh H (1998) β-Carotene hydroxylase gene from the cyanobacterium Synechocystis sp. strain PCC6803. Plant Cell Physiol 39:560–564
Miki W (1991) Biological functions and activities of animal carotenoids. Pure Appl Chem 63:141–146
Misawa N (2010) Carotenoids. In: Mander L, Lui HW (eds) Comprehensive natural products II chemistry and biology, vol 1. Elsevier, Oxford, pp 733–753
Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol 172:6704–6712
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol 177:6575–6584
Nakagawa M, Misawa N (1991) Analysis of carotenoid glycosides produced in gram-negative bacteria by introduction of the Erwinia uredovora carotenoid biosynthesis genes. Agric Biol Chem 55:2147–2148
Nishino H, Tokuda H, Murakoshi M, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Okuda M, Khachik F, Narisawa T, Takasuka N, Yano M (2000) Cancer prevention by natural carotenoids. Biofactors 13:89–94
Osawa A, Harada H, Choi SK, Misawa N, Shindo K (2011) Production of caloxanthin 3′-β-d-glucoside, zeaxanthin β-d-diglucoside, and nostoxanthin in a recombinant Escherichia coli expressing system harboring seven carotenoid biosynthesis genes, including crtX and crtG. Phytochemistry 72:711–716
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Seo YB, Choi SS, Nam SW, Lee JH, Kim YT (2009) Cloning and characterization of the zeaxanthin glucosyltransferase gene (crtX) from the astaxanthin-producing marine bacterium, Paracoccus haeundaensis. J Microbiol Biotechnol 19:1542–1546
Shindo K, Asagi E, Sano A, Hotta Y, Minemura N, Mikami K, Tamesada E, Misawa N, Maoka T (2008a) Diapolycopenedioic acid xylosyl esters A, B, and C, novel antioxidative glyco-C30-carotenoic acids produced by a new marine bacterium Rubritalea squalenifaciens. J Antibiot 61:185–191
Shindo K, Endo M, Miyake Y, Wakasugi K, Morritt D, Bramley PM, Fraser PD, Kasai H, Misawa N (2008b) Methyl glucosyl-3,4-dehydro-apo-8′-lycopenoate, a novel antioxidative glyco-C30-carotenoic acid produced by a marine bacterium Planococcus maritimus. J Antibiot 61:729–735
Sugiura M, Nakamura M, Ogawa K, Ikoma Y, Yano M (2012) High serum carotenoids associated with lower risk for bone loss and osteoporosis in post-menopausal Japanese female subjects: prospective cohort study. PLoS One 7:e52643
Takaichi S, Maoka T, Masamoto K (2001) Myxoxanthophyll in Synechocyctis sp. PCC 6803 is myxol 2′-dimethyl-fucoside, (3R,2′S)-myxol 2′-(2,4-di-O-methyl-α-l-fucoside), not rhamnoside. Plant Cell Physiol 42:756–762
Talegawkar SA, Johnson EJ, Carithers TC, Taylor HA Jr, Bogle ML, Tucker KL (2008) Carotenoid intakes, assessed by food-frequency questionnaires (FFQs), are associated with serum carotenoid concentrations in the Jackson Heart Study: validation of the Jackson Heart Study Delta NIRI Adult FFQs. Public Health Nutr 11:989–997
Tatsuzawa H, Maruyama T, Misawa N, Fujimori K, Nakano M (2000) Quenching of singlet oxygen by carotenoids produced in Escherichia coli-attenuation of singlet oxygen-mediated bacterial killing by carotenoids. FEBS Lett 484:280–284
Williams GJ, Goff RD, Zhang C, Thorson JS (2008) Optimizing glycosyltransferase specificity via “hot spot” saturation mutagenesis presents a catalyst for novobiocin glycorandomization. Chem Biol 15:393–401
Yokoyama A, Sandmann G, Hoshino T, Adachi K, Sakai M, Shizuri Y (1995) Thermozeaxanthins, new carotenoid-glycoside-esters from thermophilic bacterium Thermus thermophilus. Tetrahedron Lett 36:4901–4904
Yokoyama A, Shizuri Y, Misawa N (1998) Production of new carotenoids, astaxanthin glucosides, by Escherichia coli transformants carrying carotenoid biosynthetic genes. Tetrahedron Lett 39:3709–3712
Acknowledgments
The authors are grateful to the Marine Biotechnology Institute (MBI), Kamaishi-shi, Iwate, Japan (closed on June 30, 2008), since this work was initially in part performed here. S.K.C. thanks Mr. Satoru Matsuda of MBI for HPLC analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, SK., Osawa, A., Maoka, T. et al. 3-β-Glucosyl-3′-β-quinovosyl zeaxanthin, a novel carotenoid glycoside synthesized by Escherichia coli cells expressing the Pantoea ananatis carotenoid biosynthesis gene cluster. Appl Microbiol Biotechnol 97, 8479–8486 (2013). https://doi.org/10.1007/s00253-013-5101-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5101-9