Abstract
The release of acetic acid due to deacetylation of the hemicellulose fraction during the treatment of lignocellulosic biomass contributes to the inhibitory character of the generated hydrolysates. In the present study, we identified a strain-independent adaptation protocol consisting of pre-cultivating the strain at pH 5.0 in the presence of at least 4 g L−1 acetic acid that enabled aerobic growth and improved fermentation performance of Saccharomyces cerevisiae cells at low pH (3.7) and in the presence of inhibitory levels of acetic acid (6 g L−1). During anaerobic cultivation with adapted cells of strain TMB3500, the specific ethanol production rate was increased, reducing the fermentation time to 48 %.
Similar content being viewed by others
Introduction
Hemicelluloses consist of heteropolysaccharides whose main biological role is to reinforce the plant cell wall (Scheller and Ulvskov 2010). Regardless of the type of biomass, a common and important structural feature of hemicelluloses is that some hydroxyl groups of the pyranose units are substituted by acetyl groups. For example, in the galactoglucomannans found in softwood (that can represent up to 20 % of the hemicellulose fraction), there is one acetyl group for every five pyranose units, whereas xylose-based hemicelluloses from hardwood contain seven acetyl residues per ten xylose units (Sjöström 1993). When the lignocellulosic biomass is treated to obtain monomeric fermentable sugars from the cellulose and hemicellulose fractions, acetic acid is generated from the degradation of the acetylated sugars (Tran and Chambers 1985). Typically, concentrations of acetic acid ranging from 1 to 10 g L−1 can be found after the pretreatment and hydrolysis steps (Mills et al. 2009).
Acetic acid, in its undissociated form, enters the cell by passive diffusion, mainly through the aquaglyceroporin Fps1p, the open glycerol channel of the plasma membrane (Mollapour et al. 2008). Once inside the cell, acetic acid dissociates, due to a higher internal pH, generating free protons. In order to maintain the intracellular pH, cells pump out the excess of protons at the expense of at least 1 mole of ATP per mole of proton (Pampulha and Loureiro-Dias 2000). For given total concentration, the undissociated fraction of acetic acid (which can be calculated using the Henderson–Hasselbalch equation (Atkins and de Paula 2002)) increases when the pH of the cultivation decreases. For example, at pH 5.0, 42 % of the acetic acid present in the fermentation media will be undissociated, whereas the undissociated form will increase drastically to 88 % at pH 4.0, i.e. well below the acetic acid pKa (4.85, at 30 °C). As a consequence for the cell, more ATP will be required to maintain the intracellular pH at the expense of biomass formation (Pampulha and Loureiro-Dias 2000).
Ethanolic fermentations from lignocellulosic biomass performed with Saccharomyces cerevisiae are usually carried out around pH 5 to allow an efficient fermentation at the optimal pH for the microorganism (Verduyn et al. 1990). However, the lack of sterility at industrial scale level represents an extra factor of contamination of microorganisms other than the organism/strain of choice. Several studies have reported the isolation of bacterial contaminants in ethanol plants, which reduces the overall yeast fermentation performance (Hynes et al. 1997; Narendranath et al. 1997; Schell et al. 2007). Bacterial contaminants in ethanol plants are strongly inhibited at pH below 5.0 (Kádár et al. 2007). Therefore, a possible strategy to overcome the contamination issue would be to reduce the pH of the fermentation. However, the presence of weak acids—and notably acetic acid—increases the inhibitory character of the hydrolysates when the pH is decreased, decreasing yeast growth and fermentation capabilities (Maiorella et al. 1983; Narendranath et al. 2001). Therefore, the tolerance for acetic acid at low pH is one of the challenges in the development and use of industrial S. cerevisiae strains for ethanolic fermentation of lignocellulosic biomass.
In this study, we report and discuss the positive effect of pre-exposing cells to acetic acid at pH 5.0 on subsequent yeast growth and fermentation performance at low pH and in the presence of acetic acid.
Materials and methods
Strains and maintenance
Three S. cerevisiae strains were used in the study: TMB3500 (Almeida et al. 2009), baker’s yeast (BY) isolate from commercial baker’s yeast (Kronjäst Blå, Jästbolaget AB, Rotebro, Sweden) and the laboratory strain CEN.PK113-7D (EUROSCARF, Frankfurt, Germany), referred in this study as CEN.PK.
Strains were maintained on YPD agar plates, containing 10 g L−1 yeast extract, 20 g L−1 peptone, 20 g L−1 glucose and 20 g L−1 agar, and stored at −80 °C in YPD medium containing 10 g L−1 yeast extract, 20 g L−1 peptone and 20 g L−1 glucose supplemented with 30 % (v/v) glycerol.
Cultivation conditions
Chemically-defined medium (Verduyn et al. 1992) supplemented with 20 g L−1 glucose and buffered with 50 mM potassium hydrogen phthalate (Hahn-Hägerdal et al. 2005) was used throughout all aerobic experiments. All media were filter-sterilized, instead of being autoclaved, to avoid changes in composition due to acetic acid evaporation. In addition, all aerobic cultures were performed at 30 °C in a rotary shake incubator (New Brunswick, Enfield, CT, USA) at 180 rpm. Cell concentrations were determined from absorbance measurements at 620 nm on samples diluted to give an optical density (OD) below 0.4 (Spectrophotometer U-1800, Hitachi, Berkshire, UK).
Aerobic cultivations
A single colony of each S. cerevisiae strain, grown until early stationary phase in 50-mL conical tubes containing 5 mL of chemically-defined medium buffered at pH 5.0, was used to inoculate a pre-culture at an initial OD of 0.5. Pre-cultures were incubated in 500-mL shake flasks, containing 50 mL of chemically-defined medium buffered at pH 5.0, and grown until end of the exponential phase. These pre-cultures were used to inoculate aerobic adaptation cultures of 50 mL of chemically-defined medium buffered at pH 5.0, containing different concentrations of acetic acid (0, 1, 2, 4 or 6 g L−1). Cells grown for either 8 or 12 h were used to inoculate 500-mL baffled shake flasks with 50 mL of chemically-defined medium containing 6 g L−1 acetic acid and buffered at pH 3.7. Aerobic cultivations were performed in at least biological duplicates. Length of lag phase was determined by extrapolation of the linear regression line (when plotting ln OD vs. time during the exponential growth phase) to the initial ln OD value.
Anaerobic fermentations
A single TMB3500 colony was used to inoculate a 250-mL baffled shake flask containing 25 mL of chemically-defined medium buffered at pH 5.0. Cells were aerobically grown until end of exponential phase. The pre-culture was then used to inoculate a subsequent aerobic adaptation culture of chemically-defined medium buffered at pH 5.0, containing 0 or 6 g L−1 acetic acid, at initial OD of 0.5. Cells were harvested either after 8 or 12 h by centrifugation and washed with 0.9 % NaCl solution. Fermentation was carried out in a 1.4-L bioreactor (Multifors, Infors AG, Bottmingen, Switzerland), at initial cell concentration of 1 g (cell dry weight) L−1 in 1 L of chemically-defined medium (Verduyn et al. 1992) containing 6 g L−1 acetic acid. Ergosterol and Tween 80 were used in the medium at final concentration of 0.01 and 0.42 g L−1, respectively. Antifoam was used at final concentration of 0.5 mL L−1 (Dow Corning Antifoam RD emulsion, VWR International Ltd., Poole, UK). The temperature was maintained at 30 °C; stirring was set at 200 rpm, and the pH was kept constant at 3.7 by addition of 3 M KOH. Anaerobic conditions were obtained by continuously sparging with 0.2 L min−1 nitrogen gas containing less than 5 ppm O2 (AGA GAS AB, Sundbyberg, Sweden) controlled by a mass flow metre (Bronkhorst Hi-Tech, Ruurlo, the Netherlands). A fermentation monitor INNOVA 1313 (LumaSense Technologies Inc., Santa Clara, CA, USA) was used to qualitatively monitor online carbon dioxide concentration in the exhaust gas. Fermentation experiments were performed in at least biological duplicates.
Metabolite analysis
Cells were quickly separated by centrifugation; the supernatant was filtered through 0.20-μm membrane filters (Toyo Roshi Kaish, Tokyo, Japan) and stored at −20 °C until analysis. Concentrations of glucose, glycerol, acetate and ethanol were determined by high-performance liquid chromatography (HPLC) (Waters, Milford, MA, USA) using an HPX-87H resin-based column (Bio-Rad, Hercules, CA, USA) preceded by a Micro-Guard Cation H guard column (Bio-Rad). Separation was performed at 45 °C with 5 mM H2SO4 at a flow rate of 0.6 mL min−1. All compounds were quantified by refractive index detection (Shimadzu, Kyoto, Japan). For each HPLC run, a seven-point calibration curve was made for each compound to calculate concentrations. Each sample was analysed at least in duplicate.
Carbon dioxide composition in the bioreactor effluent gas was regularly determined using a CP-4900 micro GC (Varian, The Netherlands) equipped with a thermal conductivity detector. Column specifications and analysis conditions were as described in Zeidan et al. (2010).
Cell dry weight was determined in triplicate by filtering 5 mL of the culture in a pre-weighed 0.45-μm pore-size membrane filter (Pall Corporation, Port Washington, NY, USA). Filters were washed with distilled water and dried for 8 min at 350 W in a microwave oven.
Relative gene expression
RNA extraction
Gene expression analysis was performed during the acetic acid adaptation culture. Cells were grown in 100 mL of chemically-defined medium buffered at pH 5.0, containing 0 or 6 g L−1 acetic acid in 1,000-mL baffled shake flasks. Cell cultures were performed in biological triplicates. Sampling was performed every 2 h by removing 10 mL from the culture. Cells, transferred in a 15-mL conical tube pre-cooled in dry ice, were immediately centrifuged at 4 °C for 4 min at 3,800 rpm, and the supernatant was discarded. Cell pellet was immediately frozen in liquid nitrogen and stored at −80 °C until extraction. Total RNA was extracted using hot acidic phenol method (Collart and Oliviero 1995). Prior to the reverse transcription step, isolated RNA was treated with RQ1 RNase-free DNase for the removal of any DNA template (Promega, Madison, WI, USA). The extracted RNA was quantified using a UV-visible light spectrophotometer (NanoDrop, Thermo Scientific, Waltham, USA).
Quantitative real-time PCR
One microgram of total RNA was reverse-transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) in a final volume of 20 μL, according to the manufacturer’s instructions. Relative quantification was performed in a LightCycler 2.0 instrument (Roche Diagnostics GmbH, Mannheim, Germany) using TaKaRa Ex Taq Hot Start version DNA polymerase (TaKaRa Biotechnology, Otsu, Shiga, Japan) and SYBR green fluorescence dye (Roche Diagnostics GmbH, Mannheim, Germany). The reaction mix (20 μL final volume) contained 1 × Ex Taq buffer, 0.2 mM dNTP mixture, 2 mM MgCl2, 0.1 mg mL−1 BSA, 0.3 mM of each primer, 1 × SYBR green fluorescent dye, 1 U Ex Taq HS polymerase and 1 μL of cDNA template. The LightCycler amplification programme is as follows: initial denaturation step for 1 min at 95 °C, followed by 45 cycles of 5 s at 56 °C (for the PMA1 gene) or 52 °C (for the HAA1 and TAF10 genes) and 25 s at 72 °C. The primers used for the target genes PMA1 and HAA1 and the reference gene TAF10 are listed in Table 1.
The quantification cycle (Cq) was determined according to the second derivative maximum method using the LightCycler software 4.1 (Roche Diagnostics GmbH, Mannheim, Germany). The amplification efficiency for each of the genes was evaluated by preparing a tenfold serial dilution of DNase-treated RNA samples and converting it into cDNA. The slope of the standard curve, prepared by plotting the Cq against the concentration, was used to calculate the amplification efficiency using the equation E = 10(1/slope) − 1. The relative expression ratio (RE) was calculated according to the Eq. 1 (Pfaffl 2001):
Results
Acetic acid tolerance of the industrial strain TMB3500
Strain TMB3500, which has previously shown tolerance towards several non-detoxified lignocellulosic hydrolysates (Almeida et al. 2009), was preliminarily tested for acetic acid tolerance under aerobic conditions. In the presence of 6 g L−1 acetic acid at pH 5, 20 g L−1 glucose in chemically-defined medium was depleted within less than 24 h. By contrast, strain TMB3500 already showed a lag phase of 60 h at pH 4.2, where 80 % of the acetic acid is found in undissociated form (data not shown). The effect of acetic acid and pH on the growth of TMB3500 was then systematically evaluated, individually and in combination, in a series of aerobic cultivations, where cells pre-grown at pH 5.0 for 8 h were used to inoculate aerobic cultures of chemically-defined medium either at pH 5.0 or 3.7, containing either 0 or 6 g L−1 of acetic acid. Cultures were inoculated at an initial OD of 0.5, and growth was followed over time.
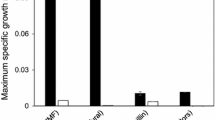
In the absence of acetic acid, the maximum specific growth rate of TMB3500 significantly decreased, from 0.47 ± 0.01 to 0.38 ± 0.00 h−1, when the pH was reduced from 5.0 to 3.7. In the presence of 6 g L−1 acetic acid, the maximum specific growth rate was reduced from 0.47 to 0.36 ± 0.01 h−1 at pH 5.0, whereas the impact was much more severe at pH 3.7 where no growth was observed even after 190 h of cultivation (Fig. 1a).
a Effect of pH and acetic acid on aerobic growth of TMB3500. TMB3500 was grown in chemically-defined medium without (open symbols) and with (solid symbols) 6 g L−1 acetic acid at pH 5.0 (open square, solid square) and pH 3.7 (open diamond, solid diamond). b Effect of adaptation on aerobic growth on synthetic medium at pH 3.7 in the presence of 6 g L−1 acetic acid. Adaptation was performed at pH 5.0 for 12 h (open circles) with 6 g L−1 acetic acid or 8 h (solid circles) with 6 g L-1 acetic acid, 4 g L−1 acetic acid (solid triangle) and 1 g L−1 acetic acid (crosses)
It has previously been shown that the lag phase observed at low pH values due to the presence of non-lethal concentrations of acetic acid could be eliminated if the cells were pre-grown until mid-exponential phase at the same pH but in the presence of lower acetic acid levels (Tenreiro et al. 2000). Here, we tested an alternative pre-cultivation strategy consisting of pre-growing cells at pH 5.0 in the presence of inhibitory concentrations of acetic acid. Indeed, cells pre-grown at pH 5.0 in the presence of 6 g L−1 acetic acid were able to grow at pH 3.7 in the presence of the same acetic acid concentration acid although at a lower maximum specific growth rate (0.11 ± 0.01 h−1) and after a lag phase of 12 ± 1 h (Fig. 1b).
Effect of acetic acid level and growth stage on cell adaptation
Different concentrations of acetic acid were tested in the pre-cultivation step at pH 5.0 to determine the minimal concentration required for cell adaptation. Pre-grown TMB3500 cells in synthetic media at pH 5.0 without acetic acid were used to inoculate aerobic adaptation cultures at pH 5.0 containing 1, 2 and 4 g L−1 of acetic acid. After 8 h, cells were harvested and used for inoculation of chemically-defined medium buffered at pH 3.7 and containing 6 g L−1 acetic acid, and growth was followed over time.
The sensitivity towards acetic acid was already observed at pH 5.0, where the maximum specific growth rate of TMB3500 decreased from 0.47 ± 0.01 to 0.42 ± 0.01 h−1 when increasing acetic acid concentration from 1 to 4 g L−1 and upwards (Table 2). This correlated with a gradual decrease in biomass yield from 0.14 ± 0.00 g biomass per gram of glucose (up to 1 g L−1 acetic acid) to 0.10 ± 0.00 g biomass per gram of glucose (4 g L−1 acetic acid and upwards) (Table 2).
During the cultivation at pH 3.7 in the presence of 6 g L−1 acetic acid, only the cells adapted at pH 5.0 with 4 g L−1 of acetic acid were able to grow at maximum specific growth rate of 0.11 ± 0.01 h−1 and after 11 ± 1 h of lag phase. Cells adapted with 1 g L−1 acetic acid did not start growing even after 150 h of cultivation (Fig. 1b) and when the adaptation culture contained 2 g L−1, a non-reproducible growth pattern was observed (data not shown).
The effect of the pre-cultivation time was also evaluated by extending the adaptation duration in the presence of 6 g L−1 acetic acid from 8 h, where cells have just arrested exponential growth, to 12 h, at the end of the growth phase on glucose. When an 8-h adaptation was performed, growth at pH 3.7 was observed after 12 ± 1 h, whereas no lag phase was observed when the adaptation was performed for 12 h (Fig. 1b). In both cases, the observed growth rate was 0.11 ± 0.01 h−1.
Anaerobic fermentations at pH 3.7 in the presence of acetic acid
To evaluate whether the adaptation step was also improving the fermentation performance, anaerobic cultures of TMB3500 at pH 3.7 in the presence of 6 g L−1 acetic acid were assessed. Cells grown at pH 5.0 for 8 h in chemically-defined medium containing either 0 or 6 g L−l acetic acid were used to inoculate, at an initial cell concentration of 1 g (cell dry weight) L−1, anaerobic bioreactors containing chemically-defined medium at pH 3.7 with 6 g L−1 acetic acid.
Cell preadaptation had a strong positive impact on the fermentation time, reducing it from 159 ± 10 h to 81 ± 2 h (Fig. 2). Glucose was immediately consumed by the adapted cells, producing almost exclusively ethanol, and the specific glucose consumption rate (0.277 ± 0.006 g glucose per gram of biomass per hour) was maintained constant over the fermentation time (Fig. 2a). In contrast, the non-adapted cells showed two differentiated consumption phases. In the first ca. 144 h, only ca. 5 g L−1 glucose was consumed, whereas in the following 15 h glucose was completely depleted (Fig. 2b). Biomass concentration slightly increased only during the second phase. The maximum specific production rate of ethanol and consumption rate of glucose for the adapted cells were 0.113 ± 0.002 g ethanol per gram of biomass per hour and 0.277 ± 0.006 g glucose per gram of biomass per hour, and therefore significantly lower than the non-adapted cells, 0.324 ± 0.023 g ethanol per gram of biomass per hour and 1.18 ± 0.14 g glucose per gram of biomass per hour, respectively (Table 3). Still, the specific ethanol production rate increased from 0.058 ± 0.002 to 0.103 ± 0.016 g ethanol per gram of biomass per hour due to a much shorter fermentation time. The ethanol yields were the same for both conditions, 0.42 ± 0.00 g ethanol per gram of consumed glucose. No further improvement in the fermentation performance was observed when the cells were adapted for 12 h instead (data not shown).
Adaptation is not strain-dependent
As strain TMB3500 is known to be tolerant to lignocellulosic hydrolysate (Almeida et al. 2009), the adaptation capability was also evaluated for other strains in order to verify whether the adaptation pattern was strain-dependent. Possible adaptation of the commercial BY and the lab strain CEN.PK113-7D (CEN.PK) were tested by pre-growing them at pH 5.0 in the presence of 6 g L−1 acetic acid for 12 h. Pre-grown cells were then used to inoculate an aerobic culture of chemically-defined medium at pH 3.7 with 6 g L−1 acetic acid at an initial OD of 0.5.
The maximum specific growth rate of BY strain, in the presence of acetic acid, was reduced from 0.37 ± 0.01 h−1 to 0.10 ± 0.02 h−1 when the pH level was decreased from 5.0 to 3.7, whereas for the CEN.PK, the reduction was from 0.32 ± 0.00 h−1 to 0.07 ± 0.01 h−1. Nevertheless, and as for the TMB3500 strain, adapted cells of BY and CEN.PK were able to grow at pH 3.7 in the presence of 6 g L−1 acetic acid, whereas non-adapted cells did not grow even after 190 h of cultivation (Fig. 3).
Aerobic cultivation of strains BY (a) and CEN.PK (b). Cells were grown in chemically-defined medium at pH 5.0 with 6 g L−1 acetic acid (solid triangle), chemically-defined media at pH 3.7 in the presence of 6 g L−1 acetic acid with cell preadaptation (solid circle) or without cell preadaptation (solid square)
Expression level of acetic acid tolerance-related genes during cell adaptation
Several genes have been reported to be involved in the mechanism of acetic acid tolerance in S. cerevisiae, most of them being related to not only “transcription” and “carbohydrate metabolism” but also “intracellular trafficking”, “ribosome biogenesis” and “nutrient sensing and response to external stimulus” (Mira et al. 2010b). Among them, PMA1 gene encodes a plasma membrane H + −ATPase whose level is increased in weak acid-challenged cells, to be able to restore the intracellular pH close to physiological value by proton extrusion (Carmelo et al. 1997). Four regulatory pathways dependent on transcription factors have been identified, to date, as mediating yeast response to weak acid stress. Among them, the transcription factor Haa1p has been suggested to be mainly responsible in the control of yeast response to acetic acid. Haa1p has been reported to specifically regulate almost one half of the genes induced by the presence of acetic acid, whereas the number of genes specifically dependent on other transcription factors involved in other weak acid adaptive response, such as Msn2p/Msn4p, Rim101p or War1p was much lower in the case of acetic acid (Mira et al. 2010a).
Therefore, HAA1 was chosen together with PMA1 and their relative gene expression levels were monitored during the adaptation step. Gene expression analyses were conducted on TMB3500 cells growing aerobically in chemically-defined medium buffered at pH 5.0 containing 0 or 6 g L−1 acetic acid and sampling was performed every 2 h. TAF1 gene was chosen as reference gene as its expression has been reported to be stable in all growth stages on glucose (Teste et al. 2009).
For the cells growing in the presence of acetic acid, an increase of induction level of PMA1 was observed after 2 h of cultivation, followed with a gradual decrease of relative expression over time (Fig. 4a). Regarding HAA1, a gradual decrease was observed until 6 h of cultivation, after which it increased again, achieving the highest level of induction after 8 h of cultivation (Fig. 4b). For the cells growing without acetic acid, the highest PMA1 relative expression level was maintained relatively constant (Fig. 4c), whereas a progressive increase of the HAA1 relative expression level was observed throughout the cultivation (Fig. 4d).
Discussion
In this work, aerobic growth and anaerobic fermentation at low pH values (3.7) in the presence of inhibitory levels of acetic acid were improved by the introduction of a short-term adaptation step consisting of pre-growing the cells in the presence of acetic acid at higher pH (5.0, in this case). The adaptation was shown to be strain-independent, although the reduction in maximal specific growth rate at a pH value well below the acetic acid pKa was more pronounced in the laboratory strain than in the industrial strains.
Improvement of growth at low pH in the presence of acetic acid has previously been achieved in S. cerevisiae using a different adaptation strategy which consisted of pre-growing the cells in the presence of sublethal acetic acid levels at low pH (Tenreiro et al. 2000). In the present work however, high level of acetic acid was critical in the adaptation step at high pH to obtain a reproducible growth pattern under aerobic conditions at pH 3.7 in the presence of 6 g L−1 of acetic acid.
The plasma membrane ATPase is known to play an important role in maintaining the internal pH as well as the ion composition inside the cell (Pampulha and Loureiro-Dias 1989), which is especially crucial when yeast cells are subjected to the presence of weak organic acids, such as acetic acid. In addition, two different adaptive responses to acetic acid have been proposed. Mollapour and Piper (2006) pinpointed intracellular acetic acid anion to be the inducer of the high-osmolarity glycerol signalling pathway. Activation of Hog1p would phosphorylate Fps1p aquaglyceroporin (Mollapour and Piper 2007), which would be the signal for endocytosis and degradation, eliminating the major channel for acetic acid diffusion inside the cell (Mollapour et al. 2008). The second hypothesis of adaptive response involves an active expulsion of acetate through plasma membrane multidrug resistance transporters, such as Tpo2p, Tpo3p and Aqr1p, whose genes are transcriptionally activated under the control of Haa1p (Fernandes et al. 2005; Tenreiro et al. 2002; Tenreiro et al. 2000). It was shown that the transcription factor Haa1p is involved in the yeast response towards the least lipophobic weak acids such as acetic acid. When compared with the Δhaa1 strain, the expression of HAA1 led to a reduction of the duration of the lag phase but not to an increase of the specific growth rate, suggesting that the role of Haa1p is essentially exercised before a cell enters the exponential growth phase (Fernandes et al. 2005). Also, Tanaka et al. (2012) recently constructed a constitutively HAA1-overexpressing S. cerevisiae strain that showed an improved growth capability at low pH in the presence of acetic acid as well as a lower intracellular acetic acid concentration compared to the wild-type strain, suggesting that the acetic acid tolerance of the HAA1-overexpressing strain would be related to a reduced intracellular accumulation of acetic acid. In the present work, the relative expression levels of PMA1 and HAA1 were followed over time during the adaptation step, while the Fps1p endocytosis hypothesis was not evaluated since the activation mechanism takes place at the protein level. Based on the obtained results, one can hypothesise that PMA1 expression levels may not be critical for the adaptation towards acetic acid since the transient overexpression after 2 h was not maintained during the whole cultivation period. On the other hand, the higher expression levels of HAA1 at the end of the adaptation would suggest that some of the genes of the Haa1p regulation are indeed activated in the diauxic transition when glucose is depleted.
Regardless of the required mechanism for the acetic acid tolerance, the presented adaptation strategy (i.e. pre-culturing the S. cerevisiae cells at optimal growth pH in the presence of acetic acid) allowed a shorter fermentation time (i.e. higher volumetric ethanol productivity) in a subsequent anaerobic fermentation. When the cells were pre-cultured in the presence of 6 g L−1 acetic acid, the specific ethanol production rate increased, reducing the fermentation time to 48 %. It has previously been demonstrated that short-term adaptation in spruce hydrolysate leads to upregulation of furaldehyde reductase activities in TMB3500 (Almeida, unpublished work). The present study suggests that the improved performances may also be related to acetic acid short-term adaptation.
References
Almeida JRM, Karhumaa K, Bengtsson O, Gorwa-Grauslund MF (2009) Screening of Saccharomyces cerevisiae strains with respect to anaerobic growth in non-detoxified lignocellulose hydrolysate. Bioresour Technol 100(14):3674–3677. doi:10.1016/j.biortech.2009.02.057
Atkins P, de Paula J (2002) Chemical equilibrium. In: de Paula J (ed) Atkins’ physical chemistry, 7th edn. Oxford University Press, Oxford, UK, pp 222–251
Carmelo V, Santos H, Sá-Correia I (1997) Effect of extracellular acidification on the activity of plasma membrane ATPase and on the cytosolic and vacuolar pH of Saccharomyces cerevisiae. Biochim Biophys Acta 1325(1):63–70. doi:10.1016/S0005-2736(96)00245-3
Chymkowitch P, Eldholm V, Lorenz S, Zimmermann C, Lindvall JM, Bjoras M, Meza-Zepeda LA, Enserink JM (2012) Cdc28 kinase activity regulates the basal transcription machinery at a subset of genes. Proc Natl Acad Sci USA 109(26):10450–10455. doi:10.1073/pnas.1200067109
Collart MA, Oliviero S (1995) Preparation of yeast RNA. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (eds) Current protocols in molecular biology, vol 2. Wiley, New York, p 13.12
Fernandes AR, Mira NP, Vargas RC, Canelhas I, Sá-Correia I (2005) Saccharomyces cerevisiae adaptation to weak acids involves the transcription factor Haa1p and Haa1p-regulated genes. Biochem Biophys Res Commun 337(1):95–103. doi:10.1016/j.bbrc.2005.09.010
Hahn-Hägerdal B, Karhumaa K, Larsson CU, Gorwa-Grauslund M, Görgens J, van Zyl WH (2005) Role of cultivation media in the development of yeast strains for large scale industrial use. Microb Cell Factories 4:31. doi:10.1186/1475-2859-4-31
Hynes SH, Kjarsgaard DM, Thomas KC, Ingledew WM (1997) Use of virginiamycin to control the growth of lactic acid bacteria during alcohol fermentation. J Ind Microbiol Biotechnol 18(4):284–291. doi:10.1038/sj.jim.2900381
Kádár Z, Maltha SF, Szengyel Z, Reczey K, de Laat W (2007) Ethanol fermentation of various pretreated and hydrolyzed substrates at low initial pH. Appl Biochem Biotechnol 137–140(1–12):847–858. doi:10.1007/s12010-007-9102-y
Maiorella B, Blanch HW, Wilke CR (1983) By-product inhibition effects on ethanolic fermentation by Saccharomyces cerevisiae. Biotechnol Bioeng 25:103–121. doi:10.1002/bit.260250109
Mills T, Sandoval N, Gill R (2009) Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol Biofuels 2(1):26. doi:10.1186/1754-6834-2-26
Mira NP, Becker JD, Sá-Correia I (2010a) Genomic expression program involving the Haa1p-regulon in Saccharomyces cerevisiae response to acetic acid. OMICS 14(5):587–601. doi:10.1089/omi.2010.0048
Mira NP, Palma M, Guerreiro JF, Sá-Correia I (2010b) Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb Cell Fact 9:79. doi:10.1186/1475-2859-9-79
Mollapour M, Piper PW (2006) Hog1p mitogen-activated protein kinase determines acetic acid resistance in Saccharomyces cerevisiae. FEMS Yeast Res 6(8):1274–1280. doi:10.1111/j.1567-1364.2006.00118.x
Mollapour M, Piper PW (2007) Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27(18):6446–6456. doi:10.1128/Mcb.02205-06
Mollapour M, Shepherd A, Piper PW (2008) Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives. Yeast 25(3):169–177. doi:10.1002/yea.1576
Narendranath NV, Hynes SH, Thomas KC, Ingledew WM (1997) Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl Environ Microbiol 63(11):4158–4163
Narendranath NV, Thomas KC, Ingledew WM (2001) Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J Ind Microbiol Biotechnol 26(3):171–177. doi:10.1038/sj/jim/7000090
Pampulha ME, Loureiro-Dias MC (1989) Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl Microbiol Biotechnol 20:286–293
Pampulha ME, Loureiro-Dias MC (2000) Energetics of the effect of acetic acid on growth of Saccharomyces cerevisiae. FEMS Microbiol Lett 184(1):69–72. doi:10.1016/S0378-1097(00)00022-7
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45. doi:10.1093/nar/29.9.e45
Schell DJ, Dowe N, Ibsen KN, Riley CJ, Ruth MF, Lumpkin RE (2007) Contaminant occurrence, identification and control in a pilot-scale corn fiber to ethanol conversion process. Bioresour Technol 98(15):2942–2948. doi:10.1016/j.biortech.2006.10.002
Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61:263–289. doi:10.1146/annurev-arplant-042809-112315
Sjöström E (1993) Wood polysaccharides. In: Sjöström E (ed) Wood chemistry, fundamentals and applications, 2nd edn. Academic, London, pp 51–70
Tanaka K, Ishii Y, Ogawa J, Shima J (2012) Enhancement of acetic acid tolerance in Saccharomyces cerevisiae by overexpression of the HAA1 gene, encoding a transcriptional activator. Appl Environ Microbiol 78(22):8161–8163. doi:10.1128/AEM.02356-12
Tenreiro S, Nunes PA, Viegas CA, Neves MS, Teixeira MC, Cabral MG, Sá-Correia I (2002) AQR1 gene (ORF YNL065w) encodes a plasma membrane transporter of the major facilitator superfamily that confers resistance to short-chain monocarboxylic acids and quinidine in Saccharomyces cerevisiae. Biochem Biophys Res Commun 292(3):741–748. doi:10.1006/bbrc.2002.6703
Tenreiro S, Rosa PC, Viegas CA, Sá-Correia I (2000) Expression of the AZR1 gene (ORF YGR224w), encoding a plasma membrane transporter of the major facilitator superfamily, is required for adaptation to acetic acid and resistance to azoles in Saccharomyces cerevisiae. Yeast 16(16):1469–1681. doi:10.1002/1097-0061(200012)16:16<1469::AID-YEA640>3.0.CO;2-A
Teste MA, Duquenne M, Francois JM, Parrou JL (2009) Validation of reference genes for quantitative expression analysis by real-time RT-PCR in Saccharomyces cerevisiae. BMC Mol Biol 10:99. doi:10.1186/1471-2199-10-99
Tran A, Chambers R (1985) Red oak wood derived inhibitors in the ethanol fermentation of xylose by Pichia stipitis CBS 5776. Biotechnol Lett 7(11):841–845. doi:10.1007/BF01025567
Verduyn C, Postma E, Scheffers WA, Van Dijken JP (1990) Energetics of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol 136(3):405–412
Verduyn C, Postma E, Scheffers WA, Van Dijken JP (1992) Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8(7):501–517. doi:10.1002/yea.320080703
Zeidan AA, Rådström P, van Niel EWJ (2010) Stable coexistence of two Caldicellulosiruptor species in a de novo constructed hydrogenproducing co-culture. Microb Cell Factories 9:102–114. doi:10.1186/1475-2859-9-102
Acknowledgments
We would like to thank Johannes Hedman, Nikoletta Zeaki and Arun Kumar for their help and support in the design and performance of the gene expression analysis and Jenny Schelin and Peter Rådström for fruitful discussions with the obtained results. The Swedish Energy Agency (Energimyndigheten) is gratefully acknowledged for financial support (projects no. 30578–1 and 35350–1).
Conflict of interests
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Sànchez i Nogué, V., Narayanan, V. & Gorwa-Grauslund, M.F. Short-term adaptation improves the fermentation performance of Saccharomyces cerevisiae in the presence of acetic acid at low pH. Appl Microbiol Biotechnol 97, 7517–7525 (2013). https://doi.org/10.1007/s00253-013-5093-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5093-5