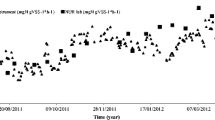
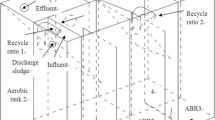
Abstract
Nitrification is an integral part of biological nitrogen removal processes and usually the limiting step in wastewater treatment systems. Since nitrification is often considered not feasible at temperatures higher than 40 °C, warm industrial effluents (with operating temperatures higher than 40 °C) need to be cooled down prior to biological treatment, which increases the energy and operating costs of the plants for cooling purposes. This study describes the occurrence of thermophilic biological nitrogen removal activity (nitritation, nitratation, and denitrification) at a temperature as high as 50 °C in an activated sludge wastewater treatment plant treating wastewater from an oil refinery. Using a modified two-step nitrification–two-step denitrification mathematical model extended with the incorporation of double Arrhenius equations, the nitrification (nitrititation and nitratation) and denitrification activities were described including the cease in biomass activity at 55 °C. Fluorescence in situ hybridization (FISH) analyses revealed that Nitrosomonas halotolerant and obligatehalophilic and Nitrosomonas oligotropha (known ammonia-oxidizing organisms) and Nitrospira sublineage II (nitrite-oxidizing organism (NOB)) were observed using the FISH probes applied in this study. In particular, this is the first time that Nitrospira sublineage II, a moderatedly thermophilic NOB, is observed in an engineered full-scale (industrial) wastewater treatment system at temperatures as high as 50 °C. These observations suggest that thermophilic biological nitrogen removal can be attained in wastewater treatment systems, which may further contribute to the optimization of the biological nitrogen removal processes in wastewater treatment systems that treat warm wastewater streams.





Similar content being viewed by others
References
Amman RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
APHA (1998) Standard methods for the examination of water and wastewater. American Public Health Association/American Water Works Association, Washington, DC
Avrahami S, Jia Z, Neufeld JD, Murrel JC, Conrad R, Küsel K (2011) Active autotrophic ammnonia-oxidizing bacteria in biofilm enrichments from simulated creek ecosystems at two ammonium concentrations respond to temperature manipulation. Appl Environ Microbiol 77(20):7329–7338
Barlindhaug J, Ødegaard H (1996) Thermal hydrolysis for the production of carbon source for denitrification. Wat Sci Tech 34(1–2): 371–378
Barrit NW (1933) The nitrification process in soils and biological filters. Ann Appl Biol. doi:10.1111/j.1744-7348.1933.tb07433
Berge ND, Reinhart DR, Dietz JD, Townsend T (2007) The impact of temperature and gas-phase oxygen on kinetics of in situ ammonia removal in bioreactor landfill leachate. Water Res 41:1907–1914
Buswell AM, Shiota T, Lawrence N, van Meter J (1954) Laboratory studies on the kinetics of the growth of Nitrosomonas with relation to the nitrification phase of the B.O.D. test. J Gral Microbiol 54:1–14
Carrera J, Vicent T, Lafuente FJ (2003) Influence of temperature on denitrification of an industrial high-strength nitrogen wastewater in a two-sludge system. Water SA 29(1):11–16
Chikamba (2009) Nitrogen removal at high temperatures: industrial applications. MSc thesis, UNESCO-IHE, Delft, The Netherlands
Choi E, Eum Y (2002) Strategy for nitrogen removal from piggery waste. Water Sci Tech 46(6–7):347–354
Christensson M, Lie E, Welander T (1994) A comparison between ethanol and methanol as carbon sources for denitrification. Water Sci Technol 30(6):83–90
Daims H, Bruh A, Amann R, Schleifer KH, Wagner M (1999) The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst Appl Microbiol 22:434–444
Daims H, Nielsen P, Nielsen JL, Juretschko S, Wagner M (2000) Novel Nitrospira-like bacteria as dominant nitrite oxidizers in biofilms from wastewater treatment plants: diversity and in situ physiology. Water Sci Technol 41:85–90
Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M (2001) In situ characterization of Nitrospira-like nitrite-oxidizing bacteria active in wastewater treatment plants. Appl Environ Microbiol 67:5273–5284
De la Torre J, Walker CB, Ingalls AE, Konneke M, Stahl DA (2008) Cultivation of a thermophilic ammonia oxidizing archaeon synthesizing crenarchaeol. Environ Microbiol 10(3):810–818
Gieseke A, Purkhold U, Wagner M, Amann R, Schramm A (2001) Community structure and activity dynamics of nitrifying bacteria in a phosphate-removing biofilm. Appl Environ Microbiol 67:1351–1362
Gilch S (2009) Molecular and physiological characterization of the energy of Nitrosomonas europaea (in German). Ph.D. dissertation, University of Bayreuth, Germany. http://opus.ub.uni-bayreuth.de/volltexte/2010/668/
Guo JH, Peng YZ, Huang HJ, Wang SY, Ge SJ, Zhang JR, Wang ZW (2010) Short- and long-term effects of temperature on partial nitrification in a sequencing batch reactor treating domestic wastewater. J Hazard Mater 179:471–479
Hellinga C, Schellen AAJC, Mulder JW, Loosdrecht MCM, Heijnen JJ (1998) The SHARON process: an innovative method for nitrogen removal from ammonium-rich waste water. Water Sci Technol 37:135–142
Hellinga C, van Loosdrecht MCM, Heijnen JJ (1999) Model-based design of a novel process for nitrogen removal from concentrated flows. Math Comput Model Dyn Syst 5(4):351–371
Henze M, Gujer W, Mino T, van Loosdrecht MCM (2000) Activated sludge models ASM1, ASM2, ASM2d and ASM3. IWA Scientific and Technical Report No. 9. IWA Task Group on Mathematical Modelling for Design and Operation of Biological Wastewater Treatment. IWA Publishing, London
Henze M, Harremoes P, Jansen JLC, Arvin E (1995) Wastewater treatment: biological and chemical processes. Springer, Berlin
Henze M, van Loosdrecht MCM, Ekama GA, Brdjanovic D (2008) Biological wastewater treatment: principles, modeling and design. IWA Publishing, London
Hiorns WD, Hastings RC, Head IM, McCarthy AJ, Saunders JR, Pickup RW, Hall GH (1996) Amplification of 16S ribosomal RNA genes of autotrophic ammonia-oxidizing bacteria demonstrates the ubiquity of nitrosospiras in the environment. Microbiololgy 141:2793–2800
Iacopozzi I, Innocenti V, Marsili-Libelli S, Giusti E (2007) A modified activated sludge model No. 3 (ASM3) with two-step nitrification–denitrification. Environ Model Softw 22:847–861
Juretschko S, Timmermann G, Schmid M, Schleifer KH, Pommerening-Roser A, Koops HP, Wagner M (1998) Conbined molecular and conventional analyses of nitrifying bacterium in activated sludge: Nitrosococcus mobilis and Nitrospira-like bacteria as dominant populations. Appl Environ Microbiol 64:3042–3051
Kartal B, Kuenen JG, van Loosdrecht MCM (2010) Sewage treatment with Anammox. Science 38:702–703
Kim J-H, Guo X, Park H-S (2008) Comparison study of the effects of temperature and free ammonia concentration on nitrification and nitrite accumulation. Process Biochem 43:154–160
Laudelout H, van Tichelen L (1960) Kinetics of the nitrite oxidation by Nitrobacter winogradskyi. J Bacteriol 79:39
Lebedeva EV, Off S, Zumbragel S, Kruse M, Shagzhina A, Lucker S, Maixner F, Lipski A, Daims H, Spieck E (2011) Isolation and characterization of a moderately thermophilic nitrite-oxidizing bacterium from a geothermal spring. FEMS Microbiol Ecol 75:195–204
Lopez-Vazquez CM, Song YI, Hooijmans CM, Brdjanovic D, Moussa MS, Gijzen HJ, van Loosdrecht MCM (2007) Short-term temperature effects on the anaerobic metabolism of glycogen accumulating organisms. Biotech Bioeng 97(3):483–495
Lopez-Vazquez CM, Hooijmans CM, Brdjanovic D, Gijzen HJ, van Loosdrecht MCM (2009) Temperature effects on glycogen accumulating organisms. Water Res 43(11):2852–2864
Lucker S, Nowka B, Rattei T, Spieck E, Daims H (2013) The genome of Nitrospina gracilis illuminates the metabolism and evolution of the major marine nitrite oxidizer. Front Microbiol 4:27
Maeda K, Morioka R, Hanajima D, Osada T (2010) The impact of using mature compost on nitrous oxide emission and the denitrifier community in the cattle manure composting process. Microb Ecol 59:25–36
Mobarry BK, Wagner M, Urbain V, Rittmann BE, Stahl DA (1996) Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol 62:2156–2162
Moussa MS, Lubberding HJ, Hooijmans CM, Van Loosdrecht MCM, Gijzen HJ (2003) Improved method for determination of ammonia and nitrite oxidation activities in mixed cultures. Appl Microbiol Biotechnol 63:217–221
Moussa MS, Rojas AR, Hooijmans CM, Gijzen HJ, van Loosdrecht MCM (2004) Model-based evaluation of nitrogen removal in a tannery wastewater treatment plant. Water Sci Technol 50:251–260
Mulder JW, van Loosdrecht MCM, Hellinga C, van Kempen R (2001) Full-scale application of the SHARON process for treatment of rejection water of digested sludge dewatering. Water Sci Technol 43(11):127–134
Nowak O, Svardal K, Schweighofer P (1995) The dynamic behaviour of nitrifying sludge systems influenced by inhibiting wastewater compounds. Water Sci Technol 31(2):115–124
Nyberg U, Aspegren H, Andersson B, Janssen JC, Villadsen IS (1992) Full-scale application of nitrogen removal with methanol as carbon source. Water Sci Technol 26(5–6):1077–1086
Oleszkiewicz JA, Berquist SA (1988) Low temperature nitrogen removal in sequencing discontinuos reactors. Water Res 22(9):1163–1171
Pinzon-Pardo AL, Brdjanovic D, Moussa MS, Lopez-Vazquez CM, Meijer SCF, Van Straten HHA, Janssen AJH, Amy G, van Loosdrecht MCM (2007) Application of ASM3 to an oil refinery wastewater treatment plant. Environ Technol 28:1273–1284
Purtschert I, Gujer W (1999) Population dynamics by methanol addition in denitrifying wastewater treatment plants. Water Sci Technol 39(1):43–50
Purtschert I, Siegrist H, Gujer W (1996) Enhanced denitrification with methanol at WWTP Zürich-Werdhölzli. Water Sci Technol 33(12):117–126
Salem S, Berends DHJG, van Loosdrecht MCM, Heijnen J (2002) Model-based evaluation of a new upgrading concept for nitrogen removal. Water Sci Technol 45(6):169–176
Shimaya, Hashimoto (2011) Isolation and characterization of novel thermophilic nitrifying Bacillus sp. from compost. Soil Sci Plant Nutr 57(1):150–156
Shore JL, McCoy WS, Gunsch CK, Deshusses MA (2012) Application of a moving bed biofilm reactor for tertiary ammonia treatment in high temperature industrial wastewater. Bioresour Technol 112:51–60
Sorokin DY, Lücker S, Vejmelkova D, Kostrikina NA, Kleerebezem R, Rijpstra WI, Sinninghe Damste JS, Le Paslier D, Muyzer G, Wagner M, Loosdrecht MCM, Daims H (2012) Nitrification expanded: discovery, physiology and genomics of a nitrite-oxidizing bacterium from the phylum Chloroflexi. ISME J 6:2245–2256
Stark JM (1996) Modeling the temperature response of nitrification. Biogeochemistry 35:433–445
Sudarno U, Winter J, Gallert C (2011) Effect of varying salinity, temperature, ammonia and nitrous acid concentrations on nitrification of saline wastewater in fixed-bed reactors. Bioresour Technol 102(10):5665–5673
Timmermans P, van Haute A (1983) Denitrification with methanol. Fundamental work of the growth and denitrification capacity of Hyphomicrobium sp. Water Res 17(10):1249–1255
Tourna M, Freitag TE, Nicol GW, Prosser JI (2008) Growth, activity and temperature responses of ammonia-oxidizing Archaea and Bacteria in soil microcosms. Environ Microbiol 10(5):1357–1364
Wagner M, Rath G, Amann R, Koops HP, Schleifer KH (1995) In-situ identification of ammonia-oxidizing bacteria. Syst Appl Microbiol 18:251–264
Wagner M, Rath G, Koops HP, Flood J, Amann R (1996) In-situ analysis of nitrifying bacteria in sewage treatment plants. Water Sci Technol 34:237–244
Wett B, Jimenez JA, Takacs I, Murthy S, Bratby JR, Holm NC, Ronner-Holm SGE (2011) Models for nitrification process design: one or two AOB populations? Water Sci Technol 66:568–578
Yamamoto N, Otawa K, Nakai Y (2010) Diversity and abundance of ammonia-oxidizing bacteria and ammonia-oxidizing Archaea during cattle manure composting. Environ Microbiol 60:807–815
Acknowledgments
The authors sincerely acknowledge the cooperation from various WWTP managers and practitioners during the collection of activated sludge samples to perform the laboratory activity tests. M. Kubare and C. Chikamba want to thank the Netherlands organization for international cooperation in higher education (Nuffic) for the financial support to undertake their M.Sc. studies at UNESCO-IHE Institute for Water Education. The valuable help provided by Markus Schmid with the confocal laser scanning microscopy of the probe-labeled Nitrospira is highly appreciated. Also, the authors would like to thank Dimitry Sorokin, from Delft University of Technology, for his valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(PDF 333 kb)
Rights and permissions
About this article
Cite this article
Lopez-Vazquez, C.M., Kubare, M., Saroj, D.P. et al. Thermophilic biological nitrogen removal in industrial wastewater treatment. Appl Microbiol Biotechnol 98, 945–956 (2014). https://doi.org/10.1007/s00253-013-4950-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4950-6