Abstract
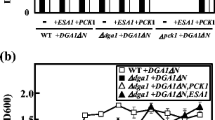
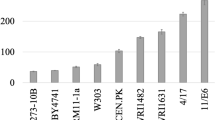
Lipid production by Saccharomyces cerevisiae was improved by overexpression of the yeast diacylglycerol acyltransferase Dga1p lacking the N-terminal 29 amino acids (Dga1∆Np), which was previously found to be an active form in the ∆snf2 mutant. Overexpression of Dga1∆Np in the ∆snf2 mutant, however, did not increase lipid content as expected, which prompted us to search for a more suitable strain in which to study the role of Dga1∆Np in lipid accumulation. We found that the overexpression of Dga1∆Np in the ∆dga1 mutant effectively increased the lipid content up to about 45 % in the medium containing 10 % glucose. The high lipid content of the transformant was dependent on glucose concentration, nitrogen limitation, and active leucine biosynthesis. To better understand the effect of dga1 disruption on the ability of Dga1∆Np to stimulate lipid accumulation, the ∆dga1-1 mutant, in which the 3′-terminal 36 bp of the dga1 open reading frame (ORF) remained, and the ∆dga1-2 mutant, in which the 3′-terminal 36 bp were also deleted, were prepared with URA3 disruption cassettes. Surprisingly, the overexpression of Dga1∆Np in the ∆dga1-1 mutant had a lower lipid content than the original ∆dga1 mutant, whereas overexpression in the ∆dga1-2 mutant led to a high lipid content of about 45 %. These results indicated that deletion of the 3′ terminal region of the dga1 ORF, rather than abrogation of genomic Dga1p expression, was crucial for the effect of Dga1∆Np on lipid accumulation. To investigate whether dga1 disruption affected gene expression adjacent to DGA1, we found that the overexpression of Esa1p together with Dga1∆Np in the ∆dga1 mutant reverted the lipid content to the level of the wild-type strain overexpressing Dga1∆Np. In addition, RT-qPCR analysis revealed that ESA1 mRNA expression in the ∆dga1 mutant was decreased compared to the wild-type strain at the early stages of culture, suggesting that lowered Esa1p expression is involved in the effect of dga1 disruption on Dga1∆Np-dependent lipid accumulation. These results provide a new strategy to engineer S. cerevisiae for optimal lipid production.





Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cao H (2011) Structure–function analysis of diacylglycerol acyltransferase sequences from 70 organisms. BMC Res Notes 4:249
Cao H, Chapital DC, Howard OD Jr, Deterding LJ, Mason CB, Shockey JM, Klasson KT (2012) Expression and purification of recombinant tung tree diacylglycerol acyltransferase 2. Appl Microbiol Biotechnol 96:711–727
Costa FF (2010) Non-coding RNAs: meet thy masters. BioEssays 32:599–608
Ejsing CS, Sampaio JL, Surendranath V, Duchoslav E, Ekroo K, Simons K, Shevchenko A (2009) Global analysis of the yeast lipidome by shotgun mass spectrometry. Proc Natl Acad Sci U S A 106:2136–2141
Geng F, Laurent BC (2004) Roles of SWI/SNF and HATs throughout the dynamic transcription of a yeast glucose-repressible gene. EMBO J 23:127–137
Hassan AH, Neely KE, Workman JL (2001) Histone acetyltransferase complexes stabilize SWI/SNF binding to promoter nucleosomes. Cell 104:817–827
Henry SA, Kohlwein SD, Carman GM (2012) Metabolism and regulation of glycerolipids in yeast Saccharomyces cerevisiae. Genetics 190:317–349
Hon K-K, Nielsen J (2012) Metabolic engineering of Saccharomyces cerevisiae: a key cell factory platform for future biorefineries. Cell Mol Life Sci 69:2671–2690
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Kamisaka Y, Yokochi T, Nakahara T, Suzuki O (1993) Characterization of the diacylglycerol acyltransferase activity in the membrane fraction from a fungus. Lipids 28:583–587
Kamisaka Y, Noda N, Tomita N, Kimura K, Kodaki T, Hosaka K (2006) Identification of genes affecting lipid content using transposon mutagenesis in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 70:646–653
Kamisaka Y, Tomita N, Kimura K, Kainou K, Uemura H (2007) DGA1 (diacylglycerol acyltransferase gene) overexpression and leucine biosynthesis significantly increase lipid accumulation in the ∆snf2 disruptant of Saccharomyces cerevisiae. Biochem J 408:61–68
Kamisaka Y, Kimura K, Uemura H, Shibakami M (2010) Activation of diacylglycerol acyltransferase expressed in Saccharomyces cerevisiae: overexpression of Dga1p lacking the N-terminal region in the ∆snf2 disruptant produces a significant increase in its enzyme activity. Appl Microbiol Biotechnol 88:105–115
Keasling JD (2010) Manufacturing molecules through metabolic engineering. Science 330:1355–1358
Kimura K, Tomita N, Uemura H, Aki T, Ono K, Kamisaka Y (2009) Improvement of stearidonic acid production in oleaginous Saccharomyces cerevisiae. Biosci Biotechnol Biochem 73:1447–1449
Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ (2001) DGAT2 is a new diacylglycerol acyltransferase gene family. J Biol Chem 276:38862–38869
Lin Y, Qi Y, Lu J, Pan X, Yuan DS, Zhao Y, Bader JS, Boeke JD (2008) A comprehensive synthetic genetic interaction network governing yeast histone acetylation and deacylation. Genes Dev 22:2062–2074
Lin Y, Lu J, Zhang J, Walter W, Dang W, Wan J, Tao S-C, Qian J, Zhao Y, Boeke JD, Berger SL, Zhu H (2009) Protein acetylation microarray reveals that NuA4 controls key metabolic target regulating gluconeogenesis. Cell 136:1073–1084
Liu Q, Siloto RMP, Snyder CL, Weselake RJ (2011) Functional and topological analysis of yeast acyl-CoA:diacylglycerol acyltransferase 2, an endoplasmic reticulum enzyme essential for triacylglycerol biosynthesis. J Biol Chem 286:13115–13126
Liu Q, Siloto RMP, Lehner R, Stone SJ, Weselake RJ (2012) Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog Lipid Res 51:350–377
Lu C, Napier JA, Clemente TE, Cahoon EB (2011) New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Curr Opin Biotechnol 22:252–259
Nevoigt E (2008) Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev 72:379–412
Ørom UA, Shiekhattar R (2011) Long non-coding RNAs and enhancers. Curr Opin Genet Dev 21:194–198
Oelkers P, Cromley D, Padamsee M, Billheimer JT, Sturley SL (2002) The DGA1 gene determines a second triglyceride synthetic pathway in yeast. J Biol Chem 277:8877–8881
Peralta-Yahya PP, Keasling JD (2010) Advanced biofuel production in microbes. Biotechnol J 5:147–162
Peterson CL, Tamkun JW (1995) The SWI–SNF complex: a chromatin remodeling machine? Trends Biochem Sci 20:143–146
Rajakumari S, Grillitsch K, Daum G (2008) Synthesis and turnover of non-polar lipids in yeast. Prog Lipid Res 47:157–171
Ratledge C (1989) Biotechnology of oils and fats. In: Ratledge C, Wilkinson SG (eds) Microbial lipids, vol 2. Academic Press, London, pp 567–668
Sandager L, Gustavsson MH, Stahl U, Dahlqvist A, Wiberg E, Banas A, Lenman M, Ronne H, Stymne S (2002) Storage lipid synthesis is non-essential in yeast. J Biol Chem 277:6478–6482
Sapountzi V, Côté J (2011) MYST-family histone acetyltransferases: beyond chromatin. Cell Mol Life Sci 68:1147–1156
Shockey JM, Gidda SK, Chapital DC, Kuan J-C, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18:2294–2313
Sherman F (2002) Getting started with yeast. Method Enzymol 350:3–41
Smith ER, Eisen A, Gu W, Sattah M, Pannuti A, Zhou J, Cook RG, Lucchesi JC, Allis CD (1998) ESA1 is a histone acetyltransferase that is essential for growth in yeast. Proc Natl Acad Sci U S A 95:3561–3565
Sorger D, Daum G (2002) Synthesis of triacylglycerol by the acyl-coenzyme A:diacy-lglycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae. J Bacteriol 184:519–524
Soukup AA, Chiang Y-M, Bok JW, Reyes-Dominguez Y, Oakley BR, Wang CCC, Strauss J, Keller NP (2012) Overexpression of the Aspergillus nidulans histone 4 acetyltransferase EsaA increases activation of secondary metabolite production. Mol Microbiol 86:314–330
Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV Jr (2004) Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem 279:11767–11776
Stone SJ, Levin MC, Farese RV Jr (2006) Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. J Biol Chem 281:40273–40282
Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV Jr (2009) The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem 284:5352–5361
Tisseur M, Kwapisz M, Morillon A (2011) Pervasive transcription—lessons from yeast. Biochimie 93:1889–1896
Uemura H (2012) Synthesis and production of unsaturated and polyunsaturated fatty acids in yeast: current state and perspectives. Appl Microbiol Biotechnol 95:1–12
Veen M, Lang C (2004) Production of lipid compounds in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 63:635–646
Voss AK, Thomas T (2009) MYST family histone acetyltransferases take center stage in stem cells and development. BioEssays 31:1050–1061
Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, Bakkoury ME, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Liebundguth N, Lockhart DJ, Lucau-Danila A, Lussier M, M’Rabet N, Menard P, Mittmann M, Pai C, Rebischung C, Revuelta JL, Riles L, Roberts CJ, Ross-MacDonald P, Scherens B, Snyder M, Sookhai-Mahadeo S, Storms R, Veronneau S, Voet M, Volckaert G, Ward TR, Wysocki R, Yen GS, Yu K, Zimmermann K, Philippsen P, Johnston M, Davis RW (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901–906
Yen C-LE, Stone SJ, Koliwad S, Harris C, Farese RV Jr (2008) DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49:2283–2301
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamisaka, Y., Kimura, K., Uemura, H. et al. Overexpression of the active diacylglycerol acyltransferase variant transforms Saccharomyces cerevisiae into an oleaginous yeast. Appl Microbiol Biotechnol 97, 7345–7355 (2013). https://doi.org/10.1007/s00253-013-4915-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4915-9