Abstract
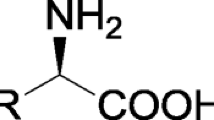
d-Branched-chain amino acids (d-BCAAs) such as d-leucine, d-isoleucine, and d-valine are known to be peptide antibiotic intermediates and to exhibit a variety of bioactivities. Consequently, much effort is going into achieving simple stereospecific synthesis of d-BCAAs, especially analogs labeled with stable isotopes. Up to now, however, no effective method has been reported. Here, we report the establishment of an efficient system for enantioselective synthesis of d-BCAAs and production of d-BCAAs labeled with stable isotopes. This system is based on two thermostable enzymes: d-amino acid dehydrogenase, catalyzing NADPH-dependent enantioselective amination of 2-oxo acids to produce the corresponding d-amino acids, and glucose dehydrogenase, catalyzing NADPH regeneration from NADP+ and d-glucose. After incubation with the enzymes for 2 h at 65°C and pH 10.5, 2-oxo-4-methylvaleric acid was converted to d-leucine with an excellent yield (>99 %) and optical purity (>99 %). Using this system, we produced five different d-BCAAs labeled with stable isotopes: d-[1-13C,15N]leucine, d-[1-13C]leucine, d-[15N]leucine, d-[15N]isoleucine, and d-[15N]valine. The structure of each labeled d-amino acid was confirmed using time-of-flight mass spectrometry and nuclear magnetic resonance analysis. These analyses confirmed that the developed system was highly useful for production of d-BCAAs labeled with stable isotopes, making this the first reported enzymatic production of d-BCAAs labeled with stable isotopes. Our findings facilitate tracer studies investigating d-BCAAs and their derivatives.




Similar content being viewed by others
References
Akita H, Fujino Y, Doi K, Ohshima T (2011) Highly stable meso-diaminopimelate dehydrogenase from an Ureibacillus thermosphaericus strain A1 isolated from a Japanese compost: purification, characterization and sequencing. AMB Express 1:43
Akita H, Doi K, Kawarabayasi Y, Ohshima T (2012) Creation of a thermostable NADP+-dependent d-amino acid dehydrogenase from Ureibacillus thermosphaericus strain A1 meso-diaminopimelate dehydrogenase by site-directed mutagenesis. Biotechnol Lett 34:1693–1699, Erratum to this report can be found in Biotechnol Lett 34:1701–1702 (2012)
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chaohui Y, Riqiang F, Jianzhi H, Lei H, Shangwu D (1993) Carbon-13 chemical shift anisotropies of solid amino acids. Magn Reson Chem 31:699–704
Chiriac M, Lupan I, Bucurenci N, Popescu O, Palibroda N (2007) Stereoselective synthesis of l-[15N] amino acids with glucose dehydrogenase and galactose mutarotase as NADH regenerating system. J Label Compd Radiopharm 51:171–174
Doveston RG, Steendam R, Jones S, Taylor RJ (2012) Total synthesis of an oxepine natural product, (±)-janoxepin. Org Lett 14:1122–1125
Ellis AV, Wilson MA (2002) Carbon exchange in hot alkaline degradation of glucose. J Org Chem 24:8469–8474
Friedman M (2010) Origin, microbiology, nutrition, and pharmacology of d-amino acids. Chem Biodivers 7:1491–1530
Friedman M, Levin CE (2012) Nutritional and medicinal aspects of d-amino acids. Amino Acids 42:1553–1582
Heine W, Wutzke K, Drescher U (1983) d-Amino acid utilization in infants measured with the 15N-tracer technique. Clin Nutr 2:31–35
Jones JG, Cottam GL, Miller BC, Sherry AD, Malloy CR (1994) A method for obtaining 13C isotopomer populations in 13C-enriched glucose. Anal Biochem 217:148–152
Katane M, Homma H (2011) d-Aspartate—an important bioactive substance in mammals: a review from an analytical and biological point of view. J Chromatogr B 879:3108–3121
Kawagishi H, Hamajima K, Takanami R, Nakamura T, Sato Y, Akiyama Y, Sano M, Tanaka O (2004) Growth promotion of mycelia of the matsutake mushroom Tricholoma matsutake by d-isoleucine. Biosci Biotechnol Biochem 68:2405–2407
Kazimierczuk K, Misiak M, Zawadzka A, Koźmiński W (2007) Progress in structural studies of proteins by NMR spectroscopy. Polimery 52:736–744
Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R (2010) d-Amino acids trigger biofilm disassembly. Science 328:627–629
Lankiewicz L, Nyassé B, Fransson B, Grehn L, Ragnarsson U (1994) Synthesis of amino acid derivatives substituted in the backbone with stable isotopes for application in peptide synthesis. J Chem Soc Perkin Trans 1:2503–2510
Martínez-Rodríguez S, Martínez-Gómez AI, Rodríguez-Vico F, Clemente–Jiménez JM, Las Heras-Vázquez FJ (2010) Natural occurrence and industrial applications of d-amino acids: an overview. Chem Biodivers 7:1531–1548
Nimura N, Kinoshita T (1986) o-Phthalaldehyde-N-acetyl-l-cysteine as a chiral derivatization reagent for liquid chromatographic optical resolution of amino acid ernantiomers and its application to conventional amino acid analysis. J Chromatogr A 352:169–177
Nishikawa T (2011) Analysis of free d-serine in mammals and its biological relevance. J Chromatogr B 879:3169–3183
Ogrel A, Shvets VI, Kaptein B, Broxterman QB, Jan R (2000) Synthesis of 15N-labelled d-isovaline and α-aminoisobutyric acid. Eur J Org Chem 2000:857–859
Ohmori T, Mutaguchi Y, Yoshikawa S, Doi K, Ohshima T (2011) Amino acid components of lees in salmon fish sauce are tyrosine and phenylalanine. J Biosci Bioeng 112:256–258
Ohshima T, Ito Y, Sakuraba H, Goda S, Kawarabayasi Y (2003) The Sulfolobus tokodaii gene ST1704 codes highly thermostable glucose dehydrogenase. J Mol Catal B Enzym 23:281–289
Panayiotis A, Karl O, Sheo BS (1984) Synthesis of (2RS)-[3-13C]- and (2RS)-[3-14C]leucine. J Label Compd Radiopharm 21:393–400
Polach KJ, Shah SA, Laluppa JC, Lemaster DM (1993) Racemic synthesis and enantiomeric conversion of [1-13C] valine. J Label Compd Radiopharm 33:809–815
Reid CM, Sutherland A (2009) 11 Synthesis of isotopically labeled α-amino acids. In: Hughes AB (ed) Amino acids, peptides and proteins in organic chemistry, volume 1, origins and synthesis of amino acids. Wiley, New Jersey, pp 473–493
Sasaki T, Takahashi S, Uchida K, Funayama S, Kainosho M, Nakagawa A (2006) Biosynthesis of quinolactacin A, a TNF production inhibitor. J Antibiot (Tokyo) 59:418–427
Scapin G, Reddy SG, Blanchard JS (1996) Three-dimensional structure of meso-diaminopimelic acid dehydrogenase from Corynebacterium glutamicum. Biochemistry 35:13540–13551
Tempelaars MH, Rodrigues S, Abee T (2011) Comparative analysis of antimicrobial activities of valinomycin and cereulide, the Bacillus cereus emetic toxin. Appl Environ Microbiol 77:2755–2762
Tiainen M, Maaheimo H, Soininen P, Laatikainen R (2010) 13C isotope effects on 1H chemical shifts: NMR spectral analysis of 13C-labelled d-glucose and some 13C-labelled amino acids. Magn Reson Chem 48:117–122
Velkov T, Thompson PE, Nation RL, Li J (2010) Structure–activity relationships of polymyxin antibiotics. J Med Chem 53:1898–1916
Wałaszek Z, Horton D, Ekiel I (1982) Conformational studies on aldonolactones by N.M.R. spectroscopy. Conformations of d-glucono-1,5-lactone and d-mannono-1,5-lactone in solution. Carbohydr Res 106:193–201
Wang W, Liu G, Yamashita K, Manabe M, Kodama H (2004) Characteristics of prolinase against various iminodipeptides in erythrocyte lysates from a normal human and a patient with prolidase deficiency. Clin Chem Lab Med 42:1102–1108
Yuan S, Ajami AM (1985) Synthesis of l-[1-13C]isoleucine via amidocarbonylation. J Label Compd Radiopharm 22:1309–1314
Yuan S, Foos J (1981) Synthesis of (S)-leucine-13C3 and its metabolites. J Label Compd Radiopharm 18:563–569
Acknowledgments
We thank Professor Seiki Kuramitsu and Ms. Keiko Ideta for TOF-MS and NMR analyses, respectively. This work was supported by a grant for Promotion of Basic Research Activities for Innovate Bioscience from the Bio-oriented Technology Research Advancement Institution (BRAIN) and Geo Biotechnology Development Organization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akita, H., Suzuki, H., Doi, K. et al. Efficient synthesis of d-branched-chain amino acids and their labeled compounds with stable isotopes using d-amino acid dehydrogenase. Appl Microbiol Biotechnol 98, 1135–1143 (2014). https://doi.org/10.1007/s00253-013-4902-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4902-1