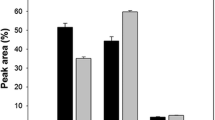
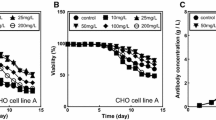
Abstract
The human host cell line, F2N78, is a new somatic hybrid cell line designed for therapeutic antibody production. To verify its potential as a human host cell line, recombinant F2N78 cells that produce antibody against rabies virus (rF2N78) were cultivated at different culture pH (6.8, 7.0, 7.2, 7.4, and 7.6) and temperatures (33.0 °C and 37.0 °C). Regardless of the culture temperature, the highest specific growth rate was obtained at a pH of 7.0–7.4. Lowering the culture temperature from 37.0 °C to 33.0 °C suppressed cell growth while allowing maintenance of high cell viability for a longer period. However, it did not enhance antibody production because specific antibody productivity did not increase at 33.0 °C. The highest maximum antibody concentration was obtained at 37.0 °C and pH 6.8. The N-linked glycosylation of the antibody was affected by the culture pH rather than the temperature. Nevertheless, G1F was dominant and G2F occupied a larger portion than G0F in all culture conditions. Compared to the same antibody produced from recombinant CHO cells, the antibody produced from rF2N78 cells has more galactose capping and was more similar to human plasma IgG. Taken together, the results obtained here demonstrate the potential of F2N78 as an alternative human host cell line for therapeutic antibody production.






Similar content being viewed by others
References
Baker KN, Rendall MH, Hills AE, Hoare M, Freedman RB, James DC (2001) Metabolic control of recombinant protein N-glycan processing in NS0 and CHO cells. Biotechnol Bioeng 73(3):188–202
Borrebaeck CK, Malmborg AC, Ohlin M (1993) Does endogenous glycosylation prevent the use of mouse monoclonal antibodies as cancer therapeutics? Immunol Today 14(10):477–479
Borys MC, Linzer DI, Papoutsakis ET (1993) Culture pH affects expression rates and glycosylation of recombinant mouse placental lactogen proteins by Chinese hamster ovary (CHO) cells. Biotechnology (N Y) 11(6):720–724
Butler M (2005) Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals. Appl Microbiol Biotechnol 68(3):283–291
Butler M (2006) Optimisation of the cellular metabolism of glycosylation for recombinant proteins produced by Mammalian cell systems. Cytotechnology 50(1-3):57–76
Cho MS, Yee H, Chan S (2002) Establishment of a human somatic hybrid cell line for recombinant protein production. J Biomed Sci 9(6 Pt 2):631–638
Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 36(1):59–74
Han YK, Ha TK, Lee SJ, Lee JS, Lee GM (2011) Autophagy and apoptosis of recombinant Chinese hamster ovary cells during fed-batch culture: effect of nutrient supplementation. Biotechnol Bioeng 108(9):2182–2192
Hossler P, Khattak SF, Li ZJ (2009) Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology 19(9):936–949
Hwang SJ, Kim SH, Kim HZ, Steinmetz MO, Koh GY, Lee GM (2008) High-level expression and purification of a designed angiopoietin-1 chimeric protein, COMP-Ang1, produced in Chinese hamster ovary cells. Protein J 27(5):319–326
Jefferis R (2009a) Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 8(3):226–234
Jefferis R (2009b) Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol Sci 30(7):356–362
Jenkins N, Curling EM (1994) Glycosylation of recombinant proteins: problems and prospects. Enzyme Microb Technol 16(5):354–364
Jenkins N, Parekh RB, James DC (1996) Getting the glycosylation right: implications for the biotechnology industry. Nat Biotechnol 14(8):975–981
Jones D, Kroos N, Anema R, van Montfort B, Vooys A, van der Kraats S, van der Helm E, Smits S, Schouten J, Brouwer K, Lagerwerf F, van Berkel P, Opstelten DJ, Logtenberg T, Bout A (2003) High-level expression of recombinant IgG in the human cell line per.c6. Biotechnol Prog 19(1):163–168
Jung ST, Reddy ST, Kang TH, Borrok MJ, Sandlie I, Tucker PW, Georgiou G (2010) Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci U S A 107(2):604–609
Kaufmann H, Mazur X, Fussenegger M, Bailey JE (1999) Influence of low temperature on productivity, proteome and protein phosphorylation of CHO cells. Biotechnol Bioeng 63(5):573–582
Kim HS, Lee GM (2007) Differences in optimal pH and temperature for cell growth and antibody production between two Chinese hamster ovary clones derived from the same parental clone. J Microbiol Biotechnol 17(5):712–720
Lee HJ, Chang MS, Kim JM, Hong HJ, Maeng KE, Koo J, Chang SJ, Cho MS (2013) Application of a new human cell line, F2N78, in the transient and stable production of recombinant therapeutics. Biotechnol Prog. doi:10.1002/btpr.1685
Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R (1996) Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol 157(11):4963–4969
Miller WM, Blanch HW, Wilke CR (1988) A kinetic analysis of hybridoma growth and metabolism in batch and continuous suspension culture: effect of nutrient concentration, dilution rate, and pH. Biotechnol Bioeng 32(8):947–965
Millward TA, Heitzmann M, Bill K, Langle U, Schumacher P, Forrer K (2008) Effect of constant and variable domain glycosylation on pharmacokinetics of therapeutic antibodies in mice. Biologicals 36:41–47
Mirick GR, Bradt BM, Denardo SJ, Denardo GL (2004) A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q J Nucl Med Mol Imaging 48(4):251–257
Muthing J, Kemminer SE, Conradt HS, Sagi D, Nimtz M, Karst U, Peter-Katalinic J (2003) Effects of buffering conditions and culture pH on production rates and glycosylation of clinical phase I anti-melanoma mouse IgG3 monoclonal antibody R24. Biotechnol Bioeng 83(3):321–334
Ozturk SS, Palsson BO (1991) Growth, metabolic, and antibody production kinetics of hybridoma cell culture: 2. Effects of serum concentration, dissolved oxygen concentration, and medium pH in a batch reactor. Biotechnol Prog 7(6):481–494
Raju TS, Briggs JB, Borge SM, Jones AJ (2000) Species-specific variation in glycosylation of IgG: evidence for the species-specific sialylation and branch-specific galactosylation and importance for engineering recombinant glycoprotein therapeutics. Glycobiology 10(5):477–486
Renard JM, Spagnoli R, Mazier C, Salles MF, Mandine E (1988) Evidence that monoclonal antibody production kinetics is related to the integral of the viable cells curve in batch systems. Biotechnol Lett 10(2):91–96
Schmid G, Blanch H, Wilke C (1990) Hybridoma growth, metabolism, and product formation in HEPES-buffered medium: II. Effect of pH. Biotechnol Lett 12(9):633–638
Stillman BW, Gluzman Y (1985) Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol 5(8):2051–2060
Trummer E, Fauland K, Seidinger S, Schriebl K, Lattenmayer C, Kunert R, Vorauer-Uhl K, Weik R, Borth N, Katinger H, Muller D (2006) Process parameter shifting: part I. Effect of DOT, pH, and temperature on the performance of Epo-Fc expressing CHO cells cultivated in controlled batch bioreactors. Biotechnol Bioeng 94(6):1033–1044
Yoon SK, Choi SL, Song JY, Lee GM (2005) Effect of culture pH on erythropoietin production by Chinese hamster ovary cells grown in suspension at 32.5 and 37.0 degrees C. Biotechnol Bioeng 89(3):345–356
Yoon SK, Hwang SO, Lee GM (2004) Enhancing effect of low culture temperature on specific antibody productivity of recombinant Chinese hamster ovary cells: clonal variation. Biotechnol Prog 20(6):1683–1688
Yoon SK, Kim SH, Lee GM (2003a) Effect of low culture temperature on specific productivity and transcription level of anti-4-1BB antibody in recombinant Chinese hamster ovary cells. Biotechnol Prog 19(4):1383–1386
Yoon SK, Song JY, Lee GM (2003b) Effect of low culture temperature on specific productivity, transcription level, and heterogeneity of erythropoietin in Chinese hamster ovary cells. Biotechnol Bioeng 82(3):289–298
Zagari F, Jordan M, Stettler M, Broly H, Wurm FM (2013) Lactate metabolism shift in CHO cell culture: the role of mitochondrial oxidative activity. N Biotechnol 30:238–245
Zamorano F, Vande Wouwer A, Jungers RM, Bastin G (2013) Dynamic metabolic models of CHO cell cultures through minimal sets of elementary flux modes. J Biotechnol 164:409–422
Acknowledgments
This research was supported in part by the WCU program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (MEST, R31-2008-000-10071-0), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 757 kb)
Rights and permissions
About this article
Cite this article
Seo, J.S., Kim, Y.J., Cho, J.M. et al. Effect of culture pH on recombinant antibody production by a new human cell line, F2N78, grown in suspension at 33.0 °C and 37.0 °C. Appl Microbiol Biotechnol 97, 5283–5291 (2013). https://doi.org/10.1007/s00253-013-4849-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4849-2