Abstract
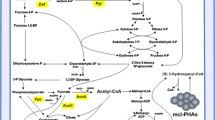
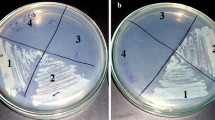
Fluorescent pseudomonads catabolize glucose simultaneously by two different pathways, namely, the oxidative pathway in periplasm and the phosphorylative pathway in cytoplasm. This study provides evidence for the role of glucose metabolism in the regulation of pyoverdine synthesis in Pseudomonas putida S11. We have characterized the influence of direct oxidation of glucose in periplasm on pyoverdine synthesis in P. putida S11. We identified a Tn5 transposon mutant of P. putida S11 showing increased pyoverdine production in minimal glucose medium (MGM). This mutant designated as IST1 had Tn5 insertion in glucose dehydrogenase (gcd) gene. To verify the role of periplasmic oxidation of glucose on pyoverdine synthesis, we constructed mutants S11 Gcd− and S11 PqqF− by antibiotic cassette mutagenesis. These mutants of P. putida S11 with loss of glucose dehydrogenase gene (gcd) or cofactor pyrroloquinoline quinone biosynthesis gene (pqqF) showed increased pyoverdine synthesis and impaired acid production in MGM. In minimal gluconate medium, the pyoverdine production of wild-type strain S11 and mutants S11 Gcd− and S11 PqqF− was higher than in MGM indicating that gluconate did not affect pyoverdine synthesis. In MGM containing PIPES–NaOH (pH 7.5) buffer which prevent pH changes due to gluconic acid production, strain S11 produced higher amount of pyoverdine similar to mutants S11 Gcd− and S11 PqqF−. Therefore, it is proposed that periplasmic oxidation of glucose to gluconic acid decreases the pH of MGM and thereby influences pyoverdine synthesis of strain S11. The increased pyoverdine synthesis enhanced biotic and abiotic surface colonization of the strain S11.







Similar content being viewed by others
References
Banin E, Vasil ML, Greenberg EP (2005) Iron and Pseudomonas aeruginosa biofilm formation. Proc Natl Acad Sci USA 102:11076–11081
Barbhaiya HB, Rao KK (1985) Production of pyoverdine, the fluorescent pigment of Pseudomonas aeruginosa PAO1. FEMS Microbiol Lett 27:233–235
Bloemberg GV, O’Toole GA, Lugtenberg BJ, Kolter R (1997) Green fluorescent protein as a marker for Pseudomonas spp. Appl Environ Microbiol 63:4543–4551
Bollinger N, Hassett DJ, Iglewski BH, Costerton JW, McDermott TR (2001) Gene expression in Pseudomonas aeruginosa: evidence of iron override effects on quorum sensing and biofilm-specific gene regulation. J Bacteriol 183:1990–1996
Cornelis P (2010) Iron uptake and metabolism in pseudomonads. Appl Microbiol Biotechnol 86:1637–1645
De Bellis P, Ercolani GL (2001) Growth interactions during bacterial colonization of seedling rootlets. Appl Environ Microbiol 67:1945–1948
de Lorenzo V, Herrero M, Jakubzik U, Timmis KN (1990) Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing, and chromosomal insertion of cloned DNA in gram-negative eubacteria. J Bacteriol 172:6568–6572
de Werra P, Pechy-Tarr M, Keel C, Maurhofer M (2009) Role of gluconic acid production in the regulation of biocontrol traits of Pseudomonas fluorescens CHA0. Appl Environ Microbiol 75:4162–4174
del Castillo T, Ramos JL, Rodriguez-Herva JJ, Fuhrer T, Sauer U, Duque E (2007) Convergent peripheral pathways catalyze initial glucose catabolism in Pseudomonas putida: genomic and flux analysis. J Bacteriol 189:5142–5152
Deziel E, Comeau Y, Villemur R (2001) Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J Bacteriol 183:1195–1204
Dorrestein PC, Poole K, Begley TP (2003) Formation of the chromophore of the pyoverdine siderophores by an oxidative cascade. Org Lett 5:2215–2217
Duffy BK, Defago G (1999) Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Appl Environ Microbiol 65:2429–2438
Duine JA (1991) Quinoproteins: enzymes containing the quinonoid cofactor pyrroloquinoline quinone, topaquinone or tryptophan-tryptophan quinone. Eur J Biochem 200:271–284
Geels FP, Schmidt EDL, Schippers B (1985) The use of 8-hydroxyquinoline for the isolation and prequalification of plant growth-stimulating rhizosphere pseudomonads. Biol Fert Soils 1:167–173
Goldstein AH (1995) Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol Agr Horticult 12:185–193
Gutterson N (1990) Microbial fungicides: recent approaches to elucidating mechanisms. Crit Rev Biotechnol 10:69–91
Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP (1998) A broad-host range Flp-FRT recombination system for site specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86
Holscher T, Gorisch H (2006) Knockout and overexpression of pyrroloquinoline quinone biosynthetic genes in Gluconobacter oxydans 621H. J Bacteriol 188:7668–7676
Hofte M, Buysens S, Koedam N, Cornelis P (1993) Zinc affects siderophore-mediated high affinity iron uptake systems in the rhizosphere Pseudomonas aeruginosa 7NSK2. Biometals 6:85–91
James HE, Beare PA, Martin LW, Lamont IL (2005) Mutational analysis of a bifunctional ferrisiderophore receptor and signal-transducing protein from Pseudomonas aeruginosa. J Bacteriol 187:4514–4520
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and L-tryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant-Microbe Interact 19:250–256
Kaur R, Macleod J, Foley W, Nayudu M (2006) Gluconic acid: an antifungal agent produced by Pseudomonas species in biological control of take-all. Phytochemistry 67:595–604
Kilstrup M, Kristiansen KN (2000) Rapid genome walking: a simplified oligo-cassette mediated polymerase chain reaction using a single genome-specific primer. Nucleic Acids Res 28:e55
Kraus J, Loper JE (1995) Characterization of a genomic region required for production of the antibiotic pyoluteorin by the biological control agent Pseudomonas fluorescens Pf-5. Appl Environ Microbiol 61:849–854
Lemanceau P, Bauer P, Kraemer S, Briat JF (2009) Iron dynamics in the rhizosphere as a case study for analyzing interactions between soils, plants and microbes. Plant Soil 321:513–535
Lenhoff H (1963) An inverse relationship of the effects of oxygen and iron on the production of fluorescein and cytochrome c by Pseudomonas fluorescens. Nature 199:601–602
Lessie TG, Phibbs PV Jr (1984) Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol 38:359–387
Loper JE, Henkels MD (1999) Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl Environ Microbiol 65:5357–5363
Lugtenberg BJ, Kravchenko LV, Simons M (1999) Tomato seed and root exudate sugars: composition, utilization by Pseudomonas biocontrol strains and role in rhizosphere colonization. Environ Microbiol 1:439–446
Martin VJ, Mohn WW (1999) An alternative inverse PCR (IPCR) method to amplify DNA sequences flanking Tn5 transposon insertions. J Microbiol Methods 35:163–166
Marugg JD, Nielander HB, Horrevoets AJ, van Megen I, van Genderen I, Weisbeek PJ (1988) Genetic organization and transcriptional analysis of a major gene cluster involved in siderophore biosynthesis in Pseudomonas putida WCS358. J Bacteriol 170:1812–1819
Meyer JM (2000) Pyoverdines: pigments, siderophores and potential taxonomic markers of fluorescent Pseudomonas species. Arch Microbiol 174:135–142
Mirleau P, Philippot L, Corberand T, Lemanceau P (2001) Involvement of nitrate reductase and pyoverdine in competitiveness of Pseudomonas fluorescens strain C7R12 in soil. Appl Environ Microbiol 67:2627–2635
Molina MA, Godoy P, Ramos-Gonzalez MI, Munoz N, Ramos JL, Espinosa-Urgel M (2005) Role of iron and the TonB system in colonization of corn seeds and roots by Pseudomonas putida KT2440. Environ Microbiol 7:443–449
Nowak-Thompson B, Gould SJ, Kraus J, Loper JE (1994) Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can J Microbiol 40:1064–1066
O’Toole GA, Kolter R (1998) Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461
Ponraj P, Shankar M, Ilakkiam D, Gunasekaran P (2012) Influence of siderophore pyoverdine synthesis and iron-uptake on abiotic and biotic surface colonization of Pseudomonas putida S11. Biometals 25(6):1113–1128. doi:10.1007/s10534-012-9574-2
Robin A, Mazurier S, Mougel C, Vansuyt G, Corberand T, Meyer JM, Lemanceau P (2007) Diversity of root-associated fluorescent pseudomonads as affected by ferritin overexpression in tobacco. Environ Microbiol 9:1724–1737
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sashidhar B, Podile AR (2010) Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol 109:1–12
Schalk IJ, Hannauer M, Braud A (2011) New roles for bacterial siderophores in metal transport and tolerance. Environ Microbiol 13:2844–285455
Schleissner C, Reglero A, Luengo JM (1997) Catabolism of D-glucose by Pseudomonas putida U occurs via extracellular transformation into D-gluconic acid and induction of a specific gluconate transport system. Microbiology 143:1595–1603
Schnider U, Keel C, Voisard C, Defago G, Haas D (1995) Tn5-directed cloning of pqq genes from Pseudomonas fluorescens CHA0: mutational inactivation of the genes results in overproduction of the antibiotic pyoluteorin. Appl Environ Microbiol 61:3856–3864
Schnider-Keel U, Seematter A, Maurhofer M, Blumer C, Duffy B, Gigot-Bonnefoy C, Reimmann C, Notz R, Defago G, Haas D, Keel C (2000) Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol 182:1215–1225
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Visca P, Imperi F, Lamont IL (2007) Pyoverdine siderophores: from biogenesis to biosignificance. Trends Microbiol 15:22–30
Yeterian E, Martin LW, Guillon L, Journet L, Lamont IL, Schalk IJ (2010) Synthesis of the siderophore pyoverdine in Pseudomonas aeruginosa involves a periplasmic maturation. Amino Acids 38:1447–1459
Acknowledgments
The work was financially supported by the Indian Council for Agricultural Research (NBAIM/AMAAS/2007-2012/MG (5)/PG/BG/3), India. The authors thank H.P. Schweizer for plasmid pEX18Tc and F. Wisniewski-Dye for E. coli S17-1 λ-pir strain. Support facilities from the Centre for Advanced studies in Functional Genomics, Centre for Excellence in Genomic Sciences, UGC-Networking Resource Centre in Biological Sciences, and DBT-MKU Interdisciplinary Life Science Programme for Advanced Research and Education are gratefully acknowledged. PP gratefully acknowledges the valuable technical assistance and discussions from M. Jeya and S. Thiagarajan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponraj, P., Shankar, M., Ilakkiam, D. et al. Influence of periplasmic oxidation of glucose on pyoverdine synthesis in Pseudomonas putida S11. Appl Microbiol Biotechnol 97, 5027–5041 (2013). https://doi.org/10.1007/s00253-013-4737-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4737-9