Abstract
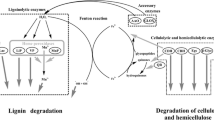
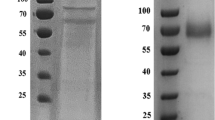
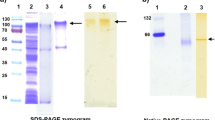
Thermobifida fusca is a moderately thermophilic soil bacterium belonging to Actinobacteria. It has been known for its capability to degrade plant cell wall polymers except lignin and pectin. To know whether it can produce enzymes to facilitate lignin degradation, the extracellular proteins bound to sugarcane bagasse were harvested and identified by liquid chromatography tandem mass spectrometry. Among the identified proteins, a putative copper-containing polyphenol oxidase of 241 amino acids, encoded by the locus Tfu_1114, was thought to presumably play a role in lignin degradation. This protein (Tfu1114) was thus expressed in E. coli and characterized. Similarly to common laccases, Tfu1114 is able to catalyze the oxidation reaction of phenolic and nonphenolic lignin related compounds such as 2,6-dimethoxyphenol and veratryl alcohol. More interestingly, it can significantly enhance the enzymatic hydrolysis of bagasse by xylanase and cellulase. Tfu1114 is stable against heat, with a half-life of 4.7 h at 90 °C, and organic solvents. It is sensitive to ethylenediaminetetraacetic acid and reducing agents but resistant to sodium azide, a potent inhibitor of laccases. Atomic absorption spectroscopy indicated that the ratio of copper to the protein monomer is 1, instead of 4, a feature of classical laccases. All these data suggest that Tfu1114 is a novel oxidase with laccase-like activity, potentially useful in biotechnology application.







Similar content being viewed by others
References
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Beloqui A, Pita M, Polaina J, Martinez-Arias A, Golyshina OV, Zumarraga M, Yakimov MM, Garcia-Arellano H, Alcalde M, Fernandez VM, Elborough K, Andreu JM, Ballesteros A, Plou FJ, Timmis KN, Ferrer M, Golyshin PN (2006) Novel polyphenol oxidase mined from a metagenome expression library of bovine rumen: biochemical properties, structural analysis, and phylogenetic relationships. J Biol Chem 281:22933–22942
Bollag JM, Leonowicz A (1984) Comparative studies of extracellular fungal laccases. Appl Environ Microbiol 48:849–854
Cheng YF, Yang CH, Liu WH (2005) Cloning and expression of Thermobifida xylanase gene in the methylotrophic yeast Pichia pastoris. Enzyme Microb Technol 37:541–546
Ducros V, Brzozowski AM, Wilson KS, Brown SH, Østergaard P, Schneider P, Yaver DS, Pedersen AH, Davies GJ (1998) Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 Å resolution. Nat Struct Biol 5:310–316
Durán N, Rosa MA, D’Annibale A, Gianfreda L (2002) Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: a review. Enzyme Microb Technol 31:907–931
Enguita FJ, Martins LO, Henriques AO, Carrondo MA (2003) Crystal structure of a bacterial endospore coat component. A laccase with enhanced thermostability properties. J Biol Chem 278:19416–19425
Ghangas GS, Hu YJ, Wilson DB (1989) Cloning of a Thermomonospora fusca xylanase gene and its expression in Escherichia coli and Streptomyces lividans. J Bacteriol 171:2963–2969
Giardina P, Faraco V, Pezzella C, Piscitelli A, Vanhulle S, Sannia G (2010) Laccases: a never-ending story. Cell Mol Life Sci 67:369–385
Hakulinen N, Kiiskinen LL, Kruus K, Saloheimo M, Paananen A, Koivula A, Rouvinen J (2002) Crystal structure of a laccase from Melanocarpus albomyces with an intact trinuclear copper site. Nat Struct Biol 9:601–605
Hullo MF, Moszer I, Danchin A, Martin-Verstraete I (2001) CotA of Bacillus subtilis is a copper-dependent laccase. J Bacteriol 183:5426–5430
Karhunen E, Niku-Paavola ML, Viikari L, Haltia T, van der Meer RA, Duine JA (1990) A novel combination of prosthetic groups in a fungal laccase; PQQ and two copper atoms. FEBS Lett 267:6–8
Kües U, Rühl M (2011) Multiple multi-copper oxidase gene families in basidiomycetes—what for? Curr Genomics 12:72–94
Kunamneni A, Ballesteros A, Plou FJ, Alcalde M (2007) Fungal laccase—a versatile enzyme for biotechnological applications. In: Méndez-Vilas A (ed) Communicating current research and educational topics and trends in applied microbiology, vol 1, Formatex Research Center. Badajoz, Spain, pp 233–245
Lykidis A, Mavromatis K, Ivanova N, Anderson I, Land M, DiBartolo G, Martinez M, Lapidus A, Lucas S, Copeland A, Richardson P, Wilson DB, Kyrpides N (2007) Genome sequence and analysis of the soil cellulolytic actinomycete Thermobifida fusca YX. J Bacteriol 189:2477–2486
Madhavi V, Lele SS (2009) Laccases: properties and applications. Biol Res 4:1684–1717
Maki M, Leung KT, Qin W (2009) The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int J Biol Sci 5:500–516
Minasov G, Shuvalova L, Mondragon A, Taneja B, Moy SF, Collart F, Anderson WF (2011) 1.8 Å crystal structure of an uncharacterized B. steaarothermophilus protein. RCSB PDB, Protein Data Bank. doi:10.2210/pdb1t8h/pdb
Moreira MT, Feijoo G, Sierra-Alvarez R, Lema J, Field JA (1997) Biobleaching of oxygen delignified kraft pulp by several white rot fungal strains. J Biotechnol 53:237–251
Mussatto SI, Dragone G, Guimaraes PM, Silva JP, Carneiro LM, Roberto IC, Vicente A, Domingues L, Teixeira JA (2010) Technological trends, global market, and challenges of bio-ethanol production. Biotechnol Adv 28:817–830
Palmieri G, Giardina P, Bianco C, Scaloni A, Capasso A, Sannia G (1997) A novel white laccase from Pleurotus ostreatus. J Biol Chem 272:31301–31307
Piontek K, Antorini M, Choinowski T (2002) Crystal structure of a laccase from the fungus Trametes versicolor at 1.90- Å resolution containing a full complement of coppers. J Biol Chem 277:37663–37669
Piscitelli A, Pezzella C, Giardina P, Faraco V, Giovanni S (2010) Heterologous laccase production and its role in industrial applications. Bioengineered Bugs 1:252–262
Reinhammar B, Malmström BG (1981) “Blue”-copper-containing oxidases. In: Spiro TG (ed) Copper proteins. Wiley-Interscience, New York, pp 109–149
Rodriguez Couto S, Toca Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Ryan S, Schnitzhofer W, Tzanov T, Cavaco-Paulo A, Gübitz GM (2003) An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enzyme Microb Technol 33:766–774
Solomon EI, Sundaram UM, Machonkin TE (1996) Multicopper oxidases and oxygenases. Chem Rev 96:2563–2606
Yang C-H, Yang S-F, Liu W-H (2007) Production of xylooligosaccharides from xylans by extracellular xylanases from Thermobifida fusca. J Agric Food Chem 55:3955–3959
Zhang S, Barr BK, Wilson DB (2000) Effects of noncatalytic residue mutations on substrate specificity and ligand binding of Thermobifida fusca endocellulase cel6A. Eur J Biochem 267:244–252
Acknowledgment
We would like to thank the National Science Council, Taiwan, for the financial support (NSC 98-2313-B-005-019-MY3).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, CY., Hsieh, ZS., Cheepudom, J. et al. A 24.7-kDa copper-containing oxidase, secreted by Thermobifida fusca, significantly increasing the xylanase/cellulase-catalyzed hydrolysis of sugarcane bagasse. Appl Microbiol Biotechnol 97, 8977–8986 (2013). https://doi.org/10.1007/s00253-013-4727-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4727-y