Abstract
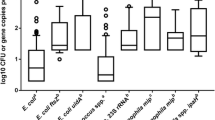
Enteric bacterial pathogens in sewage sludge easily become viable but nonculturable (VBNC) during anaerobic digestion, which escape detection by standard culture methods and pose a potential health risk. In this study, a method that is combining the standard culture method with the reverse transcription quantitative PCR (RT-qPCR) assay was developed for the quantification of bacterial pathogens in the VBNC state. The cycle threshold (C T ) values from RT-qPCR assays were linear to the bacterial number in the range from 109 to 102 most probable number (MPN) per reaction for Escherichia coli (R 2 = 0.9964) and Salmonella typhimurium (R 2 = 0.9938) and from 109 to 104 MPN per reaction for Shigella flexneri (R 2 = 0.997), respectively. Mesophilic anaerobic digestion (MAD) caused the bacterial pathogens in sewage sludge entering into VBNC state with the incidence indexes of 0.01–1.12 for E. coli, 2.48–436.52 for S. typhimurium, and 4.17–6.61 for S. flexneri, respectively. Given different VBNC incidence indexes of bacterial pathogens in sewage sludge by MAD, the quantification results of VBNC pathogens using RT-qPCR could provide an improved evaluation of pathogen inactivation efficiency and biological safety in sludge anaerobic digestion.




Similar content being viewed by others
References
CDC (Chinese Center for Disease Control and Prevention) (2008) Microbiological examination of food hygiene. Enumeration of Escherichia coli. GB/T 4789.38. Standards Press, Beijing
Chen Y, Fu B, Wang Y, Jiang Q, Liu H (2012) Reactor performance and bacterial pathogen removal in response to sludge retention time in a mesophilic anaerobic digester treating sewage sludge. Bioresour Technol 106:20–26
Cunningham E, O’Byrne C, Oliver JD (2009) Effect of weak acids on Listeria monocytogenes survival: evidence for a viable but nonculturable state in response to low pH. Food Control 20(12):1141–1144
Dinu LD, Bach S (2011) Induction of viable but nonculturable Escherichia coli O157:H7 in the phyllosphere of lettuce: a food safety risk factor. Appl Environ Microbiol 77(23):8295–8302
Fischer-Le Saux M, Hervio-Heath D, Loaec S, Colwell RR, Pommepuy M (2002) Detection of cytotoxin-hemolysin mRNA in nonculturable populations of environmental and clinical Vibrio vulnificus strains in artificial seawater. Appl Environ Microbiol 68(11):5641–5646
Gregory JB, Litaker RW, Noble RT (2006) Rapid one-step quantitative reverse transcriptase PCR assay with competitive internal positive control for detection of enteroviruses in environmental samples. Appl Environ Microbiol 72(6):3960–3967
Henry DP, Frost AJ, Samuel JL, O’Boyle DA, Thomson RH (1983) Factors affecting the survival of Salmonella and Escherichia coli in anaerobically fermented pig waste. J Appl Bacteriol 55(1):89–95
Hu JH, Li JL, Ma ZF, Lu L (2007) Detection of Shigella in milk by polymerase chain reaction (PCR) and real-time PCR. Food Sci 28(8):433–437
Higgins MJ, Chen YC, Murthy SN, Hendrickson D, Farrel J, Schafer P (2007) Reactivation and growth of non-culturable indicator bacteria in anaerobically digested biosolids after centrifuge dewatering. Water Res 41(3):665–673
Jacobsen CS, Holben WE (2007) Quantification of mRNA in Salmonella sp. seeded soil and chicken manure using magnetic capture hybridization RT-PCR. J Microbiol Methods 69(2):315–321
Jolivet-Gougeon A, Sauvager F, Bonnaure-Mallet M, Colwell RR, Cormier M (2006) Virulence of viable but nonculturable S. typhimurium LT2 after peracetic acid treatment. Int J Food Microbiol 112(2):147–152
Kunte DP, Yeole TY, Ranade DR (2000) Effect of volatile fatty acids on Shigella dysenteriae during anaerobic digestion of human night soil. World J Microbiol Biotechnol 16:519–522
Liu YM, Gilchrist A, Zhang J, Li XF (2008) Detection of viable but nonculturable Escherichia coli O157:H7 bacteria in drinking water and river water. Appl Environ Microbiol 74(5):1502–1507
Liu YM, Wang C, Fung C, Li XF (2010) Quantification of viable but nonculturable Escherichia coli O157:H7 by targeting the rpoS mRNA. Anal Chem 82(7):2612–2615
Matsuda K, Tsuji H, Asahara T, Kado Y, Nomoto K (2007) Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl Environ Microbiol 73(1):32–39
Nocker A, Cheung CY, Camper AK (2006) Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J Microbiol Methods 67(2):310–320
Oliver JD (2010) Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev 34(4):415–425
Puchajda B, Oleszkiewicz J (2006) Extended acid digestion for inactivation of fecal coliforms. Water Environ Res 78(12):2389–2396
Qi YN, Dentel SK, Herson DS (2008) Effect of total solids on fecal coliform regrowth in anaerobically digested biosolids. Water Res 42(14):3817–3825
Rudi K, Moen B, Dromtorp SM, Holck AL (2005) Use of ethidium monoazide and PCR in combination for quantification of viable and dead cells in complex samples. Appl Environ Microbiol 71(2):1018–1024
Sheridan GEC, Masters CI, Shallcross JA, Mackey BM (1998) Detection of mRNA by reverse transcription-PCR as an indicator of viability in Escherichia coli cells. Appl Environ Microbiol 64:1313–1318
US Environmental Protection Agency (US EPA) (1999) Environmental regulations and technology—control of pathogens and vectors in sewage sludge. EPA/625/R-92/013, Revised October, 1999
Vattakaven T, Bond P, Bradley G, Munn CB (2006) Differential effects of temperature and starvation on induction of the viable-but-Nonculturable state in the coral pathogens Vibrio shiloi and Vibrio tasmaniensis. Appl Environ Microbiol 72(10):6508–6513
Viau E, Peccia J (2009) Evaluation of the enterococci indicator in biosolids using culture-based and quantitative PCR assays. Water Res 43(19):4878–4887
Wang L, Li Y, Mustapha A (2007) Rapid and simultaneous quantitation of Escherichia coli O157:H7, Salmonella, and Shigella in ground beef by multiplex real-time PCR and immunomagnetic separation. J Food Prot 70(6):1366–1372
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 50978124) and the Fundamental Research Funds for the Central Universities of China (nos. JUSRP31105 and JUSRP111A10).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 78 kb)
Rights and permissions
About this article
Cite this article
Jiang, Q., Fu, B., Chen, Y. et al. Quantification of viable but nonculturable bacterial pathogens in anaerobic digested sludge. Appl Microbiol Biotechnol 97, 6043–6050 (2013). https://doi.org/10.1007/s00253-012-4408-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4408-2