Abstract
A process for human influenza H1N1 virus vaccine production from Madin–Darby canine kidney (MDCK) cells using a novel packed-bed bioreactor is described in this report. The mini-bioreactor was used to study the relationship between cell density and glucose consumption rate and to optimize the infection parameters of the influenza H1N1 virus (A/New Caledonia/20/99). The MDCK cell culture and virus infection were then monitored in a disposable perfusion bioreactor (AmProtein Current Perfusion Bioreactor) with proportional–integral–derivative control of pH, dissolved O2 (DO), agitation, and temperature. During 6 days of culture, the total cell number increased from 2.0 × 109 to 3.2 × 1010 cells. The maximum virus titers of 768 hemagglutinin units/100 μL and 7.8 × 107 50 % tissue culture infectious doses/mL were obtained 3 days after infection. These results demonstrate that using a disposable perfusion bioreactor for large-scale cultivation of MDCK cells, which allows for the control of DO, pH, and other conditions, is a convenient and stable platform for industrial-scale production of influenza vaccines.
Similar content being viewed by others
Introduction
Influenza vaccines have traditionally been prepared in chicken eggs (Robertson et al. 1995; Nichol 2003). However, there are problems associated with the use of eggs in the vaccine manufacturing process, including low efficiency, time-consuming procedures and variability in their susceptibility to influenza infection (Monto et al. 1981; Fedson 2008). Therefore, the rapid production of high-quality influenza vaccines will be an important goal to achieve in the coming decades.
In contrast to growing influenza viruses in eggs, cell culture is far more amenable to large-scale production, and the use of continuous cell lines to generate viral vaccines offers several advantages, including the opportunity to use fully characterized and standardized cells substrates. In addition, a cell culture-derived vaccine does not require extensive advance planning and can be produced rapidly on a large scale in the event of an emerging pandemic (WHO 1995). The most prominent adherent mammalian cell lines for influenza vaccine development are the commercially available African green monkey kidney (Vero) cells and Madin–Darby canine kidney (MDCK) cells, which have been extensively studied (Genzel et al. 2010; Liu et al. 2009; Romanova et al. 2004; Nicolson et al. 2005; Le Ru et al. 2010; Genzel et al. 2006b; Bock et al. 2010). MDCK cells are particularly favored, because they can yield high quantities of influenza virus. As MDCK cells are adherent in culture, a select number of large-scale culture systems are available for using these cells. Such systems include hollow fiber membrane bioreactor, rotary cell culture system, packed-bed bioreactor (PBR), and microcarrier culture (Genzel et al. 2006a; Golmakany et al. 2005; Sun and Zhang 2007; Chen and Palmer 2009; Schwarz et al. 1992). The microcarrier culture system was introduced in 1967 and has been widely used for suspension culture of anchor-dependent cells (van Wezel 1967). The system has many advantages over roller bottles and other static cultures for large-scale cultivation of anchorage-dependent cells, including being easy to monitor and control, scale-up capacity, efficient gas–liquid oxygen transfer, and space-saving design. Despite these advantages, the microcarrier culture system also has some limitations in terms of the need for sterilization and validation of the microcarrier and cleaning procedures in the stirred tank bioreactor. In addition, cells attached to the microcarrier surface frequently become damaged, which can be associated with the impellor shear force and friction force on the bioreactor wall. The PBR is a new and potentially improved option for influenza virus production in terms of scaling up, high oxygen transfer, and high yields.
In this work, we describe a process for human influenza H1N1 virus vaccine production from MDCK cells cultured in serum-containing Dulbecco’s modified eagle medium (DMEM) using a novel PBR system. We used the glucose consumption rate (GCR) as an indirect method to estimate cell growth and density in the AmProtein Current Perfusion Bioreactor (ACPB) with proportional–integral–derivative control of pH, dissolved O2 (DO), agitation, and temperature. The influenza H1N1 viruses grew to high titers (peak titer of 7.8 × 107 50 % tissue culture infectious doses per milliliter (TCID50/mL)), suggesting that this ACPB-based cell culture system is favorable for the production of influenza vaccines in MDCK cells.
Materials and methods
Cell lines
Adherent MDCK cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, CCL-34) and grown at 37 °C in high-glucose DMEM (Gibco-12800-082) supplemented with 5 % fetal bovine serum (FBS; Min Hai Bioengineering, People’s Republic of China) and 3.7 g/L NaHCO3 (Beijing Beihua Fine Chemicals Co., Ltd., People’s Republic of China).
Virus
The egg-adapted human influenza H1N1 strain (A/New Caledonia/20/99) was passaged in MDCK cells containing DMEM medium without serum supplemented with TPCK-treated trypsin (T1426, Sigma, St. Louis, MO) three times before use. Virus production was measured by titration and expressed as viral hemagglutinin (HA) units or TCID50/mL. The seed virus stock was stored in 10-mL aliquots (6.5 × 107 TCID50/mL) at −80 °C.
Roller bottle and T-flask cultures
Cell expansions were performed by recovering the frozen stock from the liquid nitrogen cell bank and thawing the cells directly into a T-75 flask (Corning, Lowell, MA) containing DMEM with 5 % FBS. After 3 days, these cells were subcultured in T-150 flasks (working volume of 50 mL). Cultivation was carried out at 37 °C in a 5 % CO2 incubator. Roller bottle cultures (850 cm2) were inoculated with approximately 2.5 × 107 cells and grown for 3 days in the same medium. When fully confluent (1.0–1.2 × 108 cells), the cells were washed three times with phosphate buffered saline (PBS) before addition of DMEM supplemented with 2.5 μg/mL TPCK-treated trypsin (T1426, Sigma). The trypsin activity was stopped by addition of an equal volume of DMEM with 5 % FBS. The cell suspension was used to inoculate the ACPB culture.
Mini-bioreactor vessel experiments
The AmProtein self-rotating mini-bioreactor was packed with 0.6 g of polymer fiber paper carriers for optimization of growth conditions (Fig. 1). To study the relationship between cell density and GCR, 14 mini-bioreactor vessels were seeded with approximately 6 × 106 cells in 30-mL complete medium and incubated at 37 °C in a 5 % CO2. A stirring speed was maintained at 45 rpm. Medium in the mini-bioreactor was exchanged daily with fresh DMEM. Two mini-bioreactor vessels were removed each day to determine the cell density and glucose concentration.
To determine the effect of different densities of MDCK cells in the mini-bioreactors, four groups were seeded with approximately 2 × 106, 4 × 106, 6 × 10 6, or 8 × 106 cells in 30-mL complete medium and incubated at 37 °C in a 5 % CO2 incubator. The medium in the mini-bioreactor was changed daily with fresh DMEM. Each group of cells was set up in duplicate, and the mean value was calculated. Samples were taken from the mini-bioreactors at 12-h intervals during the cell growth and virus production stages.
Optimization of infection parameters in mini-bioreactor vessels
To determine effects of the inoculum and TPCK-treated trypsin on influenza virus vaccine production from MDCK cells in the mini-bioreactor vessels, eight groups were seeded with approximately 2 × 106 cells in 30-mL complete medium. The medium in the mini-bioreactor vessels was replaced daily with fresh DMEM. After 5 days, the cells were washed three times with 30 mL of PBS and resuspended in 30 mL of fresh DMEM. In one set of cells, the medium was supplemented with 2.5 μg/mL TPCK-treated trypsin and inoculated with A/New Caledonia/20/99 at four different multiplicities of infection (MOI = 0.5, 0.1, 0.05, and 0.01). In another set of cells, 30 mL of fresh DMEM medium was inoculated with the virus at one dose (MOI = 0.05), while the concentration of TPCK-treated trypsin varied (1, 2.5, 5, and 10 μg/mL). Each group of cells was set up in duplicate, and the mean value was determined. Samples from the mini-bioreactor vessels were taken at 24-h intervals during cell growth.
ACPB culture
The perfusion column of the ACPB was pre-filled with 150 g of single-use polymer fiber carriers (Fig. 2a). PBS (20 mM, 6 L) was pumped through the sterile perfusion column overnight. After replacing the PBS from the ACPB with cell growth medium, cells (5 × 108 cells/L, 4 L total) from roller bottles were added to a feed bottle, transferred into the perfusion column, and incubated for 1 h without rocking. The ACPB reactor was set to a temperature of 37 °C with a rocking rate of 55 rpm, and the pH level was maintained at 7.4 by sparging CO2 and addition of 7.5 % NaHCO3. The circulation rate was 150 mL/min. Dissolved O2 was maintained at 40 % of air saturation by sparging air and N2. An illustration of the ACPB is provided in Fig. 2b.
After 6 days of culture and before virus addition, the cell growth medium was removed, and the ACPB was washed three times with 4 L of PBS. Viruses (MOI = 0.05) in 10 L of fresh DMEM supplemented with 2.5 μg/mL TPCK-treated trypsin were pumped into the reactor. For the virus infection and propagation, bioreactor operating conditions were similar to those during the growth of MDCK cells, except that the temperature was maintained at 34 °C.
Sampling and analytics
Samples from the ACPB bioreactor were taken at 12-h intervals during cell growth and the virus production step. Cell concentrations and viability in roller bottles and T-flasks were measured using the Trypan Blue dye exclusion method with a hemacytometer. Cell concentrations in the mini-bioreactor vessels and ACPB were measured using crystal violet staining. Glucose concentrations were determined using an YSI 7100 Biochemistry Analyzer (YSI Incorporated Life Sciences, USA). HA titrations were conducted in 96-well microplates using turkey red blood cells according to standard procedures (WHO 2002). Virus infectious titers were determined as TCID50 in MDCK cells (WHO 2002).
Results
Cell expansion and preparation for ACPB cell inoculation
To evaluate their stability, MDCK cells were cultured over 20 passages in 75 cm2 flasks. The cell concentrations and doubling times were similar over these passages (data not shown). A seed train process was developed using different sized T-flasks and roller bottles (Table 1). This process ensures the number of cells seeded actually exceeded that required for efficient expansion.
Determination of GCR in mini-bioreactor vessels
AmProtein (Hangzhou, China) has developed a small disposable (plastic) rolling bioreactor system to evaluate the culture parameters before scaling up to the ACPB system based on polymer fiber paper carriers. Because the culture volume of the mini-bioreactor (30 mL) was relatively small, MDCK cells consumed nutrients quickly and required frequent replacement of culture supplements. Based on our experience, daily replacement of one volume of the culture medium for MDCK cells in the growth phase was required in order to maintain the residual glucose level above 5.5 mM.
The GCR of the MDCK cell culture in the mini-bioreactor vessels was determined daily based on the residual glucose concentration and the incoming fresh medium glucose concentration, which expressed in mM of glucose consumed per day and per gram of polymer fiber paper carriers (mMg−1 day−1). Profiles of the cell growth and GCRs in the mini-bioreactor culture vessels of MDCK cells are shown in Fig. 3a. Cell growth was directly related to the GCR. In our experiments, we determined by direct cell counts from a number of cultures that approximately 1 × 108 cells corresponded to a GCR of 1.48 mMg−1 day−1 with polymer fiber paper carriers. At 6 days after seeding, the total cell number increased more than tenfold from 1 × 107 to 1.2 × 108 cells.
Profiles of cell growth and GCR in the mini-bioreactor vessel culture of MDCK cells. a Cell density (white square) is expressed in cell counts per gram of polymer fiber paper carriers, and GCR (black square) is expressed in mM of glucose consumed per day and per grams of polymer fiber paper carriers. b GCR levels for different seeding cell densities in the mini-bioreactor vessels: 2 × 106 cells (black square), 4 × 106 cells (white triangle), 6 × 106 cells (white square), 8 × 106 cells (black triangle)
In comparing the effects of different cell seeding densities in the mini-bioreactor vessels, no influence on the maximum GCR was observed (Fig. 3b). The same maximum GCR was reached in all the mini-bioreactors although at different time points. The seeding density of 8 × 106 cells per 0.6 g of polyester disks was considered most appropriate for the culture time.
Optimization of infection parameters in mini-bioreactor vessels
To optimize influenza virus yields, effects of trypsin and inoculation dose (MOI from 0.01 up to 0.5) in the mini-bioreactor were investigated (Fig. 4a). When MDCK cells were infected at a high MOI of 0.5 in the mini-bioreactor vessel, viruses in the culture medium could still be detected by the HA assay after infection. By comparison, when the MDCK cells were infected at the MOI of 0.1 or lower, no virus could be detected at the same time. With the initial MOI of 0.5, an increase in the virus titer was observed for only 12 h postinoculation, while the HA titer remained at 128 HA units/100 μL. The same trend was observed at the MOI of 0.1, which also resulted in a low titer (256 HA/100 μl). Higher virus titers of 512 and 384 HA units/100 μL were achieved for the lower MOI of 0.05 and 0.01, respectively.
Optimization of the infection parameters in mini-bioreactor vessels. a Influence of different MOI on virus yield in the mini-bioreactor vessels: MOI 0.01(white), 0.05 (light gray), 0.1 (gray), 0.5 (black). b Influence of different trypsin concentrations (0, 1, 2.5, 5, 10 µg/ml) on virus yield in the mini-bioreactor vessels
The concentration of trypsin had a significant effect on virus titer (Fig. 4b). Without trypsin, the virus titer was undetectable in the culture medium, even 4 days postinfection. When trypsin was added to the MDCK cells at the concentration of 5 μg/mL, a low virus titer of 256 HA units/100 μL was reached. For the higher trypsin concentrations of 1 and 2.5 μg/mL, the virus titer was similar at 512 HA units/100 μL. Consequently, the MOI of 0.05 corresponding to the highest virus titer achieved with the trypsin concentration of 2.5 μg/mL was used for virus expansion in the ACPB.
ACPB culture
MDCK cells from roller bottles were seeded in the first ACPB cell culture run (ACPB-1) at 5 × 108 cells/L (4 L). During the first hour, cells were maintained under quiescent conditions to allow attachment. Circulation was initiated at a flow rate of 100 mL/min and then increased stepwise to 200 and 300 mL/min in 30-min intervals. These experiments showed that about 95 % of the seed cells attached during the 1-h quiescent period in the ACPB. During the subsequent propagation phase, the circulation was at a flow rate of 400 mL/min. The GCR profiles in the ACPB cultures of MDCK cells are given in Fig. 5a. The perfusion was started at 48 h when the glucose decreased to 6 mM, and daily replacement of 50 % of the MDCK culture medium in the growth phase was required in order to maintain the glucose level above 5.5 mM. The final cell density of 2.2 × 108/g (150 g) was estimated from the specific GCR at 120 h. By crystal violet staining of fiber carriers taken from the ACPB, the total number of MDCK cells was determined to be 3.2 × 1010. The cell densities obtained by these two different methods were similar. Furthermore, our analysis of the cell density at different positions suggested a stable and even distribution pattern throughout the perfusion column (Fig. 5b).
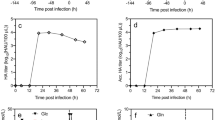
In the next run (ACPB-2), the influence of harvest time on the virus titer in the ACPB was evaluated every 12 h during the infection period (Fig. 6a). Virus titers could be quantified by HA assays after a delay of 24 h after infection. The maximum virus yield of 512 HA units/100 μL was reached at 2 days postinfection and remained stable. Meanwhile, the TCID50 value of 7.3 × 107/mL was reached at 96 h. To test the reproducibility of the HA titer and TCID50 in the ACPB, additional runs were carried out in the same culture conditions and infection parameters. At 3 days postinoculation, the maximum HA titers of 768 and 512 and TCID50 values of 7.8 × 107 and 6.7 × 107/mL were reached in the subsequent experimental runs ACPB-3 and ACPB-4, respectively (Fig. 6b).
Discussion
In this study, we developed a process for the production of human influenza H1N1 virus vaccine based on the use of a disposable PBR. Since cells in the PBR are immobilized during culture and are not accessible for direct sampling/counting, a reliable indirect method for estimating cell density in this system was developed. Normally, cell densities can be estimated by the O2 uptake rate (Dorresteijn et al. 1996; Eyer and Heinzle 1996; Fassnacht and Portner 1999), GCR (Kaufman et al. 2000; Meuwly et al. 2006), glutamine consumption (Yang and Butler 2000), and lactate production rate (Cruz et al. 1999; Sun and Zhang 2003). In this study, GCR was used as an indirect indicator to estimate cell growth and density in the APCB. MDCK cells were first cultured in mini-bioreactors to study the relationship between cell density and GCR. Crystal violet staining and counts with a hemacytometer were also used to determine the final cell density in the mini-bioreactor. The results obtained were consistent between the two different methods, verifying the applicability of GCR in the estimation of cell density and tracing cell growth in PBRs.
The trypsin concentration is a critical factor for efficient production of influenza vaccines from MDCK cells. Trypsin significantly enhances the infectivity of influenza virus by cleaving HA into the HA1 and HA2 subunits, which is a necessary step in the initial viral entry process. MDCK and Vero cells have been found to release protease inhibitors into the cell culture medium, which can affect the activity of trypsin (Kaverin and Webster 1995). In our study, no influenza particles were detected in the culture medium even after 3 days without trypsin. Since an excess of trypsin in the virus production medium can result in cell detachment, it is important to determine the appropriate concentration. We found that the addition of 2.5 μg/mL trypsin to the mini-bioreactor culture had a significant enhancing effect on the production of influenza virus within 3 days.
The inoculum can influence virus growth characteristics and is another important factor for efficient production of influenza vaccines from MDCK cells (Schulze-Horsel et al. 2009). Infection of cells at an excessively high MOI would increase the proportion of empty or noninfectious virus particles, while an MOI that is too low would decrease virus production. In this study, the MOI of 0.05 corresponding to the maximum virus yield achieved in the mini-bioreactor vessels was selected to be used with a trypsin concentration of 2.5 μg/mL in the ACPB cultures.
PBRs for the cultivation of immobilized mammalian cells have been widely used for many years (Golmakany et al. 2005; Meuwly et al. 2004; Kaufman et al. 2000). Carriers constructed of nonwoven polyester and polypropylene screens, spheres or other shapes of ceramic, and glass fibers have been considered suitable packing materials. Bioreactor manufacturers have developed commercial PBRs that can accommodate many different types of carriers. The CelliGen® bioreactor (New Brunswick Scientific, USA) was primarily developed for use in combination with Fibra-Cel® polyester disk carriers and has been scaled-up from 0.7 to 40 L of packed-bed volume. The TideCell® bioreactors (CESCO Bioengineering Co. Ltd, Taiwan) have also been developed with internal PBRs with BioNOC II® polyester strips carriers in a 10–50 L volume. Among these carriers, the Fibra-Cel® is quite popular for the manufacture of monoclonal antibodies and vaccines. The CelliGen® bioreactor is packed with 6-mm diameter Fibra-Cel® disks (composed of nonwoven polyester fiber and polypropylene) within a basket inside the bioreactor vessels. The mixing of cells in the culture is performed by an impellor, while the oxygen transfer is conducted through air-sparging at the bottom of the vessel. Unfortunately, a major drawback of such PBR systems is their relatively small volume, which is not practical for industrial applications such as manufacturing of vaccines.
While most commercial PBRs have internal configurations, the ACPB (AP20C) has an external recirculation system for the culture medium, which includes a bioreactor bag and perfusion column with working volumes of 10 and 5 L, respectively (Fig. 2c). This perfusion column consists of a plastic bag filled with a paper carrier for culturing high-density adherent cells. The paper carrier is constructed from polymer fibers and shaped into a nonwoven material designed with a shape, thickness, and density suitable for cell attachment and growth as well as medium circulation and perfusion. The bioreactor bag is filled with medium and then placed on the shaking platform. A system controller is configured to monitor and control the DO level, pH level, and temperature of the culture medium inside the bioreactor bag. The system controller can also adjust the rate of the rotor and the speed of the pump to achieve the desired cell culture conditions.
The ACPB based on a non-sparging O2 transfer method is used as a DO generator to culture immobilized cells in a perfusion column filled with a nonwoven polymer fiber carrier. During the cell culture, mixed gas is continually passed through the headspace utilizing the sterilizing filters provided on the bioreactor bag. The gentle shaking motion of the bioreactor bag provides a bubble-free oxygenated culture medium which flows into an inlet of the perfusion column by a peristaltic pump, passes through the paper carrier to which MDCK cells are attached, and exits at an outlet of the perfusion column to the inlet of the bioreactor bag for recirculation through the system. The shaking rate and gas flow are optimized by the system controller to provide an oxygenated culture medium for high-density cell culture in the perfusion column without excessive foaming or shear damage. Meanwhile, using serum-containing DMEM in microcarrier cultivations for influenza production requires time-consuming steps for washing and medium exchange. Compared with cell suspension perfusion culture using an expensive hollow fiber column, perfusion culture in the ACPB is much more affordable and scalable. Although using serum-free medium can eliminate the need for washing cells before infection, cell growth is generally not as robust as that in serum-containing medium. Compared with microcarrier cultures, using serum-containing DMEM for the production of influenza vaccine in the ACPB has definite advantages in terms of the reduced cost and need for cleaning/validation.
In the ACPB, the bioreactor bag and perfusion column are constructed with pre-sterilized plastic. This design eliminates the need for cleaning, sterilization and associated validation, and, thus, shortens the implementation time to conform to good manufacturing practices (GMP). The gamma radiation-sterilized ACPB reduces the risk of contamination due to equipment malfunction or operator error typical of traditional bioreactors such as stirred tanks, spinners, and hollow-fiber systems. Once a cultivation cycle is completed, the culture is harvested, and a new ACPB can immediately replace the discarded one on the shaking platform, which will greatly improve the capacity of vaccine production. By changing different specifications, the ACPB can easily be scaled up to 300 L with a larger perfusion column and bioreactor bag.
In summary, a process for using serum-containing DMEM for the production of influenza vaccine in the ACPB is presented. The mini-bioreactor was used to study the relationship between cell density and GCR and to optimize the parameters for production of influenza H1N1 virus (A/New Caledonia/20/99). After optimization, the total cell number was found to increase from 2.0 × 109 to 3.2 × 1010 cells after 6 days in the ACPB, and the maximum virus titers of 768 HA units/100 μL and 107.8 TCID50/mL were obtained 3 days after influenza infection at an MOI of 0.05. Consequently, we conclude that the disposable ACPB, with a disposable design that can reduce the need for cleaning/validation as well as the time for implementation in the GMP environment, may be a suitable bioreactor system for industrial manufacturing of influenza vaccines.
References
Bock A, Schulze-Horsel J, Schwarzer J, Rapp E, Genzel Y, Reichl U (2010) High-density microcarrier cell cultures for influenza virus production. Biotechnol Prog. doi:10.1002/btpr.539
Chen G, Palmer AF (2009) Hemoglobin-based oxygen carrier and convection enhanced oxygen transport in a hollow fiber bioreactor. Biotechnol Bioeng 102(6):1603–1612. doi:10.1002/bit.22200
Cruz HJ, Moreira JL, Carrondo MJ (1999) Metabolic shifts by nutrient manipulation in continuous cultures of BHK cells. Biotechnol Bioeng 66(2):104–113. doi:10.1002/(SICI)1097-0290(1999)66:2<104::AID-BIT3>3.0.CO;2-#
Dorresteijn RC, Numan KH, de Gooijer CD, Tramper J, Beuvery EC (1996) On-line estimation of the biomass activity during animal-cell cultivations. Biotechnol Bioeng 51(2):206–214. doi:10.1002/(SICI)1097-0290(19960720)51:2<206::AID-BIT10>3.0.CO;2-K
Eyer K, Heinzle E (1996) On-line estimation of viable cells in a hybridoma culture at various DO levels using ATP balancing and redox potential measurement. Biotechnol Bioeng 49(3):277–283. doi:10.1002/(SICI)1097-0290(19960205)49:3<277::AID-BIT5>3.0.CO;2-H
Fassnacht D, Portner R (1999) Experimental and theoretical considerations on oxygen supply for animal cell growth in fixed-bed reactors. J Biotechnol 72(3):169–184
Fedson DS (2008) NEW technologies for meeting the global demand for pandemic influenza vaccines. Biologicals 36(6):346–349. doi:10.1016/j.biologicals.2008.07.001
Genzel Y, Fischer M, Reichl U (2006a) Serum-free influenza virus production avoiding washing steps and medium exchange in large-scale microcarrier culture. Vaccine 24(16):3261–3272. doi:10.1016/j.vaccine.2006.01.019
Genzel Y, Olmer RM, Schafer B, Reichl U (2006b) Wave microcarrier cultivation of MDCK cells for influenza virus production in serum containing and serum-free media. Vaccine 24(35–36):6074–6087. doi:10.1016/j.vaccine.2006.05.023
Genzel Y, Dietzsch C, Rapp E, Schwarzer J, Reichl U (2010) MDCK and Vero cells for influenza virus vaccine production: a one-to-one comparison up to lab-scale bioreactor cultivation. Appl Microbiol Biotechnol 88(2):461–475. doi:10.1007/s00253-010-2742-9
Golmakany N, Rasaee MJ, Furouzandeh M, Shojaosadati SA, Kashanian S, Omidfar K (2005) Continuous production of monoclonal antibody in a packed-bed bioreactor. Biotechnol Appl Biochem 41(Pt 3):273–278. doi:10.1042/BA20040121
Kaufman JB, Wang G, Zhang W, Valle MA, Shiloach J (2000) Continuous production and recovery of recombinant Ca2+ binding receptor from HEK 293 cells using perfusion through a packed bed bioreactor. Cytotechnology 33(1–3):3–11. doi:10.1023/A:1008143132056
Kaverin NV, Webster RG (1995) Impairment of multicycle influenza virus growth in Vero (WHO) cells by loss of trypsin activity. J Virol 69(4):2700–2703
Le Ru A, Jacob D, Transfiguracion J, Ansorge S, Henry O, Kamen AA (2010) Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing. Vaccine 28(21):3661–3671. doi:10.1016/j.vaccine.2010.03.029
Liu J, Shi X, Schwartz R, Kemble G (2009) Use of MDCK cells for production of live attenuated influenza vaccine. Vaccine 27(46):6460–6463. doi:10.1016/j.vaccine.2009.06.024
Meuwly F, von Stockar U, Kadouri A (2004) Optimization of the medium perfusion rate in a packed-bed bioreactor charged with CHO cells. Cytotechnology 46(1):37–47. doi:10.1007/s10616-005-2105-z
Meuwly F, Papp F, Ruffieux PA, Bernard AR, Kadouri A, von Stockar U (2006) Use of glucose consumption rate (GCR) as a tool to monitor and control animal cell production processes in packed-bed bioreactors. J Biotechnol 122(1):122–129. doi:10.1016/j.jbiotec.2005.08.005
Monto AS, Maassab HF, Bryan ER (1981) Relative efficacy of embryonated eggs and cell culture for isolation of contemporary influenza viruses. J Clin Microbiol 13(1):233–235
Nichol KL (2003) The efficacy, effectiveness and cost-effectiveness of inactivated influenza virus vaccines. Vaccine 21(16):1769–1775
Nicolson C, Major D, Wood JM, Robertson JS (2005) Generation of influenza vaccine viruses on Vero cells by reverse genetics: an H5N1 candidate vaccine strain produced under a quality system. Vaccine 23(22):2943–2952. doi:10.1016/j.vaccine.2004.08.054
Robertson JS, Cook P, Attwell AM, Williams SP (1995) Replicative advantage in tissue culture of egg-adapted influenza virus over tissue-culture derived virus: implications for vaccine manufacture. Vaccine 13(16):1583–1588
Romanova J, Katinger D, Ferko B, Vcelar B, Sereinig S, Kuznetsov O, Stukova M, Erofeeva M, Kiselev O, Katinger H, Egorov A (2004) Live cold-adapted influenza A vaccine produced in Vero cell line. Virus Res 103(1–2):187–193. doi:10.1016/j.virusres.2004.02.032
Schulze-Horsel J, Schulze M, Agalaridis G, Genzel Y, Reichl U (2009) Infection dynamics and virus-induced apoptosis in cell culture-based influenza vaccine production—flow cytometry and mathematical modeling. Vaccine 27(20):2712–2722. doi:10.1016/j.vaccine.2009.02.027
Schwarz RP, Goodwin TJ, Wolf DA (1992) Cell culture for three-dimensional modeling in rotating-wall vessels: an application of simulated microgravity. J Tissue Cult Methods 14(2):51–57
Sun XM, Zhang YX (2003) Estimation of Chinese hamster ovary cell density in packed-bed bioreactor by lactate production rate. Biotechnol Lett 25(11):853–857
Sun X, Zhang Y (2007) A cell-detaching reactor for inoculation of anchorage-dependent CHO and Vero cells between stepwise-expanded bioreactors. Biotechnol Lett 29(5):697–701. doi:10.1007/s10529-006-9294-1
van Wezel AL (1967) Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 216(5110):64–65
WHO (1995) Cell culture as a substrate for the production of influenza vaccines: memorandum from a WHO meeting. Bull World Health Organ 73(4):431–435
WHO (2002) WHO manual on animal influenza diagnosis and surveillance. World Health Organisations, Geneva
Yang M, Butler M (2000) Enhanced erythropoietin heterogeneity in a CHO culture is caused by proteolytic degradation and can be eliminated by a high glutamine level. Cytotechnology 34(1–2):83–99. doi:10.1023/A:1008137712611
Acknowledgments
This work received financial support from the National S&T Major Project (2012zx10001-009-002) and the National High Technology Research and Development Program of China (863 Program, 2010AA022902). We thank AmProtein (Hangzhou, China) for the photo of the ACPB bioreactor and Thi Sarkis for editorial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, B., Yu, X., Kong, W. et al. Production of influenza H1N1 vaccine from MDCK cells using a novel disposable packed-bed bioreactor. Appl Microbiol Biotechnol 97, 1063–1070 (2013). https://doi.org/10.1007/s00253-012-4375-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4375-7