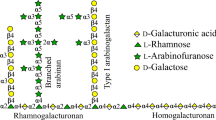
Abstract
An endo-β-1,4-galactanase (PcGAL1) and an exo-β-1,4-galactanase (PcGALX35C) were purified from the culture filtrate of Penicillium chrysogenum 31B. Pcgal1 and Pcgalx35C cDNAs encoding PcGAL1 and PcGALX35C were isolated by in vitro cloning. The deduced amino acid sequences of PcGAL1 and PcGALX35C are highly similar to a putative endo-β-1,4-galactanase of Aspergillus terreus (70 % amino acid identity) and a putative β-galactosidase of Neosartorya fischeri (72 %), respectively. Pfam analysis revealed a “Glyco_hydro_53” domain in PcGAL1. PcGALX35C is composed of five distinct domains including “Glyco_hydro_35,” “BetaGal_dom2,” “BetaGal_dom3,” and two “BetaGal_dom4_5” domains. Recombinant enzymes (rPcGAL1 and rPcGALX35C) expressed in Escherichia coli and Pichia pastoris, respectively, were active against lupin galactan. The reaction products of lupin galactan revealed that rPcGAL1 cleaved the substrate in an endo manner. The enzyme accumulated galactose and galactobiose as the main products. The smallest substrate for rPcGAL1 was β-1,4-galactotriose. On the other hand, rPcGALX35C released only galactose from lupin galactan throughout the reaction, indicating that it is an exo-β-1,4-galactanase. rPcGALX35C was active on both β-1,4-galactobiose and triose, but not on lactose, β-1,3- or β-1,6-galactooligosaccharides even after 24 h of incubation. To our knowledge, this is the first report of a gene encoding a microbial exo-β-1,4-galactanase. rPcGAL1 and rPcGALX35C acted synergistically in the degradation of lupin galactan and soybean arabinogalactan. Lupin galactan was almost completely degraded to galactose by the combined actions of rPcGAL1 and rPcGALX35C. Surprisingly, neither rPcGAL1 nor rPcGALX35C released any galactose from sugar beet pectin.




Similar content being viewed by others
References
Bauer S, Vasu P, Persson S, Mort AJ, Somerville CR (2006) Development and application of a suite of polysaccharide-degrading enzymes for analyzing plant cell walls. Proc Natl Acad Sci USA 103:11417–11422
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bonnin E, Lahaye M, Vigouroux J, Thibault JF (1995) Preliminary characterization of a new exo-β-(1,4)-galactanase with transferase activity. Int J Biol Macromol 17:345–351
Brake AJ, Merryweather JP, Coit DG, Heberlein UA, Masiarz FR, Mullenbach GT, Urdea MS, Valenzuela P, Barr PJ (1984) α-Factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 81:4642–4646
Carey AT, Smith DL, Harrison E, Bird CR, Gross KC, Seymour GB, Tucker GA (2001) Down-regulation of a ripening-related β-galactosidase gene (TBG1) in transgenic tomato fruits. J Exp Bot 52:663–668
Christgau S, Sandal T, Kofod LV, Dalbøge H (1995) Expression cloning, purification and characterization of a β-1,4-galactanase from Aspergillus aculeatus. Curr Genet 27:135–141
Cid M, Pedersen HL, Kaneko S, Coutinho PM, Henrissat B, Willats WGT, Boraston AB (2010) Recognition of the helical structure of β-1,4-galactan by a new family of carbohydrate-binding modules. J Biol Chem 285:35999–36009
Fedorova ND, Khaldi N, Joardar VS, Maiti R, Amedeo P, Anderson MJ, Crabtree J, Silva JC, Badger JH, Albarraq A, Angiuoli S, Bussey H, Bowyer P, Cotty PJ, Dyer PS, Egan A, Galens K, Fraser-Liggett CM, Haas BJ, Inman JM, Kent R, Lemieux S, Malavazi I, Orvis J, Roemer T, Ronning CM, Sundaram JP, Sutton G, Turner G, Venter JC, White OR, Whitty BR, Youngman P, Wolfe KH, Goldman GH, Wortman JR, Jiang B, Denning DW, Nierman WC (2008) Genomic islands in the pathogenic filamentous fungus Aspergillus fumigatus. PLoS Genet 4:e1000046
Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, Baştürkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D'Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M, Selker EU, Archer DB, Peñalva MA, Oakley BR, Momany M, Tanaka T, Kumagai T, Asai K, Machida M, Nierman WC, Denning DW, Caddick M, Hynes M, Paoletti M, Fischer R, Miller B, Dyer P, Sachs MS, Osmani SA, Birren BW (2005) Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105–1115
Husain Q (2010) β Galactosidases and their potential applications: a review. Crit Rev Biotechnol 30:41–62
Ichinose H, Yoshida M, Kotake T, Kuno A, Igarashi K, Tsumuraya Y, Samejima M, Hirabayashi J, Kobayashi H, Kaneko S (2005) An exo-β-1,3-galactanase having a novel β-1,3-galactan-binding module from Phanerochaete chrysosporium. J Biol Chem 280:25820–25829
Ishimaru M, Smith DL, Mort AJ, Gross KC (2009) Enzymatic activity and substrate specificity of recombinant tomato β-galactosidases 4 and 5. Planta 229:447–456
Knox JP (1995) The extracellular matrix in higher plants. 4. Developmentally regulated proteoglycans and glycoproteins of the plant cell surface. FASEB J 9:1004–1012
Kotake T, Hirata N, Degi Y, Ishiguro M, Kitazawa K, Takata R, Ichinose H, Kaneko S, Igarashi K, Samejima M, Tsumuraya Y (2011) Endo-β-1,3-galactanase from winter mushroom Flammulina velutipes. J Biol Chem 286:27848–27854
Labavitch JM, Freeman LE, Albersheim P (1976) Structure of plant cell walls. Purification and characterization of a β-1,4-galactanase which degrades a structural component of the primary cell walls of dicots. J Biol Chem 251:5904–5910
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lahaye M, Vigouroux J, Thibault JF (1991) Endo-β-1,4-D-galactanase from Aspergillus niger var. aculeatus: purification and some properties. Carbohydr Polym 15:431–444
Maksimainen M, Hakulinen N, Kallio JM, Timoharju T, Turunen O, Rouvinen J (2011) Crystal structures of Trichoderma reesei β-galactosidase reveal conformational changes in the active site. J Struct Biol 174:156–163
Moctezuma E, Smith DL, Gross KC (2003) Effect of ethylene on mRNA abundance of three β-galactosidase gene in wild type and mutant tomato fruit. Postharv Biol Technol 28:207–217
Morita M (1965) Polysaccharides of soybean seeds, part II: a methylated arabinogalactan isolated from methylated product of “hot-water-extract” fraction of soybean seed polysaccharides. Agric Biol Chem 29:626–630
Nakano H, Takenishi S, Watanabe Y (1985) Purification and properties of two galactanases from Penicillium citrinum. Agric Biol Chem 49:3445–3454
Nakano H, Takenishi S, Kitahata S, Kinugasa H, Watanabe Y (1990) Purification and characterization of an exo-1,4-β-galactanase from a strain of Bacillus subtilis. Eur J Biochem 193:61–67
Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, Bennett J, Bowyer P, Chen D, Collins M, Coulsen R, Davies R, Dyer PS, Farman M, Fedorova N, Fedorova N, Feldblyum TV, Fischer R, Fosker N, Fraser A, García JL, García MJ, Goble A, Goldman GH, Gomi K, Griffith-Jones S, Gwilliam R, Haas B, Haas H, Harris D, Horiuchi H, Huang J, Humphray S, Jiménez J, Keller N, Khouri H, Kitamoto K, Kobayashi T, Konzack S, Kulkarni R, Kumagai T, Lafon A, Latgé JP, Li W, Lord A, Lu C, Majoros WH, May GS, Miller BL, Mohamoud Y, Molina M, Monod M, Mouyna I, Mulligan S, Murphy L, O'Neil S, Paulsen I, Peñalva MA, Pertea M, Price C, Pritchard BL, Quail MA, Rabbinowitsch E, Rawlins N, Rajandream MA, Reichard U, Renauld H, Robson GD, Rodriguez de Córdoba S, Rodríguez-Peña JM, Ronning CM, Rutter S, Salzberg SL, Sanchez M, Sánchez-Ferrero JC, Saunders D, Seeger K, Squares R, Squares S, Takeuchi M, Tekaia F, Turner G, de Aldana CR Vazquez, Weidman J, White O, Woodward J, Yu JH, Fraser C, Galagan JE, Asai K, Machida M, Hall N, Barrell B, Denning DW (2005) Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature 438:1151–1156
Okemoto K, Uekita T, Tsumuraya Y, Hashimoto Y, Kasama T (2003) Purification and characterization of an endo-β-(1->6)-galactanase from Trichoderma viride. Carbohydr Res 338:219–230
Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K, Andersen MR, Bendtsen JD, Benen JA, van den Berg M, Breestraat S, Caddick MX, Contreras R, Cornell M, Coutinho PM, Danchin EG, Debets AJ, Dekker P, van Dijck PW, van Dijk A, Dijkhuizen L, Driessen AJ, d’Enfert C, Geysens S, Goosen C, Groot GS, de Groot PW, Guillemette T, Henrissat B, Herweijer M, van den Hombergh JP, van den Hondel CA, van der Heijden RT, van der Kaaij RM, Klis FM, Kools HJ, Kubicek CP, van Kuyk PA, Lauber J, Lu X, van der Maarel MJ, Meulenberg R, Menke H, Mortimer MA, Nielsen J, Oliver SG, Olsthoorn M, Pal K, van Peij NN, Ram AF, Rinas U, Roubos JA, Sagt CM, Schmoll M, Sun J, Ussery D, Varga J, Vervecken W, van de Vondervoort PJ, Wedler H, Wösten HA, Zeng AP, van Ooyen AJ, Visser J, Stam H (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25:221–231
Ponder GR, Richards GN (1997) Arabinogalactan from Western larch, part III: alkaline degradation revisited, with novel conclusions on molecular structure. Carbohydr Polym 34:251–261
Rojas AL, Nagem RAP, Neustroev KN, Arand M, Adamska M, Eneyskaya EV, Kulminskaya AA, Garratt RC, Golubev AM, Polikarpov I (2004) Crystal structures of β-galactosidase from Penicillium sp. and its complex with galactose. J Mol Biol 343:1281–1292
Ryttersgaard C, Lo Leggio L, Coutinho PM, Henrissat B, Larsen S (2002) Aspergillus aculeatus β-1,4-galactanase: substrate recognition and relations to other glycoside hydrolases in clan GH-A. Biochemistry 41:15135–15143
Sakamoto T, Kawasaki H (2003) Purification and properties of two type-B α-L-arabinofuranosidases produced by Penicillium chrysogenum. Biochim Biophys Acta (General subjects) 1621:204–210
Sakamoto T, Thibault JF (2001) An exo-arabinanase of Penicillium chrysogenum able to release arabinobiose from α-1,5-L-arabinan. Appl Environ Microbiol 67:3319–3321
Sakamoto T, Ihara H, Kozaki S, Kawasaki H (2003) A cold-adapted endo-arabinanase from Penicillium chrysogenum. Biochim Biophys Acta (General subjects) 1624:70–75
Sakamoto T, Nishimura S, Kato T, Sunagawa Y, Tsutiyama M, Kawasaki H (2005) Efficient extraction of ferulic acid from sugar beet pulp using the culture supernatant of Penicillium chrysogenum. J Appl Glycosci 52:115–120
Sakamoto T, Tsujitani Y, Fukamachi K, Taniguchi Y, Ihara H (2010) Identification of two GH27 bifunctional proteins with β-L-arabinopyranosidase/α-D-galactopyranosidase activities from Fusarium oxysporum. Appl Microbiol Biotechnol 86:1115–1124
Sakamoto T, Ogura A, Inui M, Tokuda S, Hosokawa S, Ihara H, Kasai N (2011a) Identification of a GH62 α-L-arabinofuranosidase specific for arabinoxylan produced by Penicillium chrysogenum. Appl Microbiol Biotechnol 90:137–146
Sakamoto T, Tanaka H, Nishimura Y, Ishimaru M, Kasai N (2011b) Characterization of an exo-β-1,3-D-galactanase from Sphingomonas sp. 24 T and its application to structural analysis of larch wood arabinogalactan. Appl Microbiol Biotechnol 90:1701–1710
Sakamoto T, Inui M, Yasui K, Hosokawa S, Ihara H (2012a) Substrate specificity and gene expression of two Penicillium chrysogenum α-L-arabinofuranosidases (AFQ1 and AFS1) belonging to glycoside hydrolase families 51 and 54. Appl Microbiol Biotechnol. doi:10.1007/s00253-012-3978-3
Sakamoto T, Inui M, Yasui K, Tokuda S, Akiyoshi M, Kobori Y, Nakaniwa T, Tada T (2012b) Biochemical characterization and gene expression of two endo-arabinanases from Penicillium chrysogenum 31B. Appl Microbiol Biotechnol 93:1087–1096
Smith DL, Gross KC (2000) A family of at least seven beta-galactosidase genes is expressed during tomato fruit development. Plant Physiol 123:1173–1183
Smith DL, Starrett DA, Gross KC (1998) A gene coding for tomato fruit β-galactosidase II is expressed during fruit ripening. Cloning, characterization, and expression pattern. Plant Physiol 117:417–423
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Tsumuraya Y, Hashimoto Y, Yamamoto S, Shibuya N (1984) Structure of L-arabino-D-galactan-containing glycoproteins from radish leaves. Carbohydr Res 134:215–228
Tsumuraya Y, Mochizuki N, Hashimoto Y, Kovác P (1990) Purification of an exo-β-(1->3)-D-galactanase of Irpex lacteus (Polyporus tulipiferae) and its action on arabinogalactan-proteins. J Biol Chem 265:7207–7215
Van den Berg MA, Albang R, Albermann K, Badger JH, Daran JM, Driessen AJ, Garcia-Estrada C, Fedorova ND, Harris DM, Heijne WH, Joardar V, Kiel JA, Kovalchuk A, Martín JF, Nierman WC, Nijland JG, Pronk JT, Roubos JA, van der Klei IJ, van Peij NN, Veenhuis M, von Döhren H, Wagner C, Wortman J, Bovenberg RA (2008) Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nat Biotechnol 26:1161–1168
Yamaguchi F, Inoue S, Hatanaka C (1995) Purification and properties of endo-β-1,4-galactanase from Aspergillus niger. Biosci Biotechnol Biochem 59:1742–1744
Yang H, Ichinose H, Yoshida M, Nakajima M, Kobayashi H, Kaneko S (2006) Characterization of a thermostable endo-β-1,4-D-galactanase from the hyperthermophile Thermotoga maritima. Biosci Biotechnol Biochem 70:538–541
Acknowledgments
This research was partially supported by a grant-in-aid for scientific research (22580091) from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakamoto, T., Nishimura, Y., Makino, Y. et al. Biochemical characterization of a GH53 endo-β-1,4-galactanase and a GH35 exo-β-1,4-galactanase from Penicillium chrysogenum . Appl Microbiol Biotechnol 97, 2895–2906 (2013). https://doi.org/10.1007/s00253-012-4154-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4154-5