Abstract
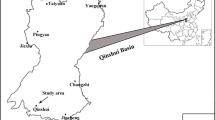
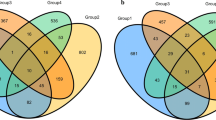
Biogenic origin of the significant proportion of coal bed methane has indicated the role of microbial communities in methanogenesis. By using cultivation-independent approach, we have analysed the archaeal and bacterial community present in the formation water of an Indian coal bed at 600–700 m depth to understand their role in methanogenesis. Presence of methanogens in the formation water was inferred by epifluorescence microscopy and PCR amplification of mcrA gene. Archaeal 16S rRNA gene clone library from the formation water metagenome was dominated by methanogens showing similarity to Methanobacterium, Methanothermobacter and Methanolinea whereas the clones of bacterial 16S rRNA gene library were closely related to Azonexus, Azospira, Dechloromonas and Thauera. Thus, microbial community of the formation water consisted of predominantly hydrogenotrophic methanogens and the proteobacteria capable of nitrogen fixation, nitrate reduction and polyaromatic compound degradation. Methanogenic potential of the microbial community present in the formation water was elucidated by the production of methane in the enrichment culture, which contained 16S rRNA gene sequences showing close relatedness to the genus Methanobacterium. Microcosm using formation water as medium as well as a source of inoculum and coal as carbon source produced significant amount of methane which increased considerably by the addition of nitrite. The dominance of Diaphorobacter sp. in nitrite amended microcosm indicated their important role in supporting methanogenesis in the coal bed. This is the first study indicating existence of methanogenic and bacterial community in an Indian coal bed that is capable of in situ biotransformation of coal into methane.





Similar content being viewed by others
References
Ahmed M, Smith JW (2001) Biogenic methane generation in the degradation of eastern Australian Permian coals. Organic Geochem 32:809–816
Altschu SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bailey JE, Ollis DF (1986) Biochemical engineering fundamentals, 2nd edn. McGraw-Hill, New York
Baker KH (1994) Bioremediation of surface and subsurface soils. In: Baker KH, Herson DS (eds) Bioremediation. McGraw-Hill, New York, pp 203–259
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Banat IM, Nedwell DB, Balba MT (1983) Stimulation of methanogenesis by slurries of saltmarsh sediment after the addition of molybdate to inhibit sulphate-reducing bacteria. J Gen Microbiol 129:123–129
Chakraborty R, Coates JD (2005) Hydroxylation and carboxylation—two crucial steps of anaerobic benzene degradation by Dechloromonas strain RCB. Appl Environ Microbiol 71:5427–5432
Chowdhury SP, Khanna S, Verma SC, Tripathi AK (2004) Molecular diversity of tannic acid degrading bacteria isolated from tannery soil. J Appl Microbiol 97:1210–1219
Coates JD, Chakraborty R, Lack JG, Connor SMO, Cole KA, Bender KS, Achenbach LA (2001) Anaerobic benzene oxidation coupled to nitrate reduction in pure culture by two strains of Dechloromonas. Nature 411:1039–1043
Cookson JT Jr (1995) Bioremediation engineering design and application. McGraw-Hill, New York
Faison BD (1992) The chemistry of low rank coal and its relationship to the biochemical mechanisms of coal biotransformation. In: Crawford DL (ed) Microbial transformations of low rank coals. CRC, Boca Raton, pp 1–26
Faiz M, Hendry P (2006) Significance of microbial activity in Australian coal bed methane reservoirs—a review. Bull Can Petrol Geol 54:261–272
Fakoussa RM, Hofrichter M (1999) Biotechnology and microbiology of coal degradation. Appl Microbiol Biotechnol 52:25–40
Flores RM, Rice CA, Stricker GD, Warden A, Ellis MS (2008) Methanogenic pathways of coalbed gas in the Powder River Basin, United States: the geologic factor. Int J Coal Geol 76:52–75
Garrity GM (ed) (2005) Bergey’s manual of systematic bacteriology, 2nd edn. Springer, New York
Gockay CF, Kolankaya N, Dilek FB (2001) Microbial solubilization of lignites. Fuel 80:1421–1433
Godon JJ, Zumstein E, Dabert P, Habouzit F, Moletta R (1997) Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl Environ Microbiol 63:2802–2813
Green MS, Flanegan KC, Gilcrease PC (2008) Characterization of a methanogenic consortium enriched from a coalbed methane well in the Powder River Basin, U.S.A. Int J Coal Geol 76:34–45
Hales BA, Edward C, Ritchie DA, Hall G, Pickup RW, Saunders JR (1996) Isolation and identification of methanogen-specific DNA from blanket bog peat by PCR amplification and sequence analysis. Appl Environ Microbiol 62:668–675
Harding R, Czarnecki S, Isbister J, Barik S (1993) Biogasification of low-rank coal. TR-101572. ARCTECH, Inc. for Electric Power Research Institute, Chantilly, p 9
Hyvik H, Eskilt JP, Hovland JS (1997) Method for removing hydrogen sulphide from oil-containing water and equipment therefor. Biotechnol Adv 15:734–735
Jin S, Fallgren P, Luo H (2010) Feasibility of enhanced biodegradation of petroleum compounds in groundwater under denitrifying conditions. Bull Environ Contam Toxicol 84:357–361
Khan ST, Hiraishi A (2002) Diaphorobacter nitroreducens gen. nov., sp. nov., a poly (3-hydroxybutyrate)-degrading denitrifying bacterium isolated from activated sludge. J Gen Appl Microbiol 48:299–308
King GM, Wiebe JM (1980) Tracer analysis of methanogenesis in salt marsh soil. Appl Environ Microbiol 39:877–881
Klankeo P, Nopcharoenkul W, Pinyakong O (2009) Two novel pyrene-degrading Diaphorobacter sp. and Pseudoxanthomonas sp. isolated from soil. J Biosci Bioengg 108:488–495
Klein DA, Flores RM, Venot C, Gabbert K, Schmid R, Stricker GD, Pruden A, Mandernac K (2008) Molecular sequences derived from Paleocene Fort Union formation coals vs. associated produced waters: implications for CBM regeneration. Int J Coal Geol 76:3–13
Kluber HD, Conrad R (1998a) Effects of nitrate, nitrite, NO and N2O on methanogenesis and other redox processes in anoxic rice field soil. FEMS Microbiol Ecol 25:301–318
Kluber HD, Conrad R (1998b) Inhibitory effects of nitrate, nitrite, NO and N2O on methanogenesis by Methanosarcina barkeri and Methanobacterium bryantii. FEMS Microbiol Ecol 25:331–339
Li D, Hendry P, Faiz M (2008) A survey of the microbial populations in some Australian coalbed methane reservoirs. Int J Coal Geol 76:14–24
LUCA Technologies, LLC (2004) Active biogenesis of methane in Wyoming’s Powder river Basin. http://www.lucatechnologies.com/content/index.cfm?fuseaction=showContent&contentID=22&navID=22
Mandal D, Tewari DC, Rautela MS (2004) Analysis of micro-fractures in coal for the coal bed methane exploitation in Jharia Coal field. Abstract 5th conference and exposition on petroleum geophysics, Hyderabad-2004, India, pp 904–909
Mazumder S, Wolf KH-AA (2004) An overview of the potential and prospects of coalbed methane exploration and exploitation in the Permo-carboniferous coal measures of the Barakar formation, Jharia basin India. Geolo Bel 7:147–156
McInerney MJ, Bryant MP (1981) Review of methane fermentation fundamentals. In: Wise DL (ed) Fuel gas production from biomass. CRC, Boca Raton, pp 19–46
Midgley DJ, Hendry P, Pinetown KL, Fuentes D, Se Gong FD, Mitchell DL, Faiz M (2010) Characterisation of a microbial community associated with a deep, coal seam methane reservoir in the Gippsland Basin, Australia. Int J Coal Geol 82:232–239
Mikucki JA, Liu Y, Delwiche M, Colwell FS, Boone DR (2003) Isolation of a methanogen from deep marine sediments that contain methane hydrates, and description of Methanoculleus submarinus sp. Nov. Appl Environ Microbiol 69:3311–3316
Mochimaru H, Yoshioka H, Tamaki H, Nakamura K, Kaneko N, Sakata S, Imachi H, Sekiguchi Y, Uchiyama H, Kamagata Y (2007a) Microbial diversity and methanogenic potential in a high temperature natural gas field in Japan. Extremophiles 11:453–461
Mochimaru H, Uchiyama H, Yoshioka H, Imachi H, Hoaki T, Tamaki H, Nakamura K, Sekiguchi Y, Kamagata Y (2007b) Methanogen diversity in deep subsurface gas-associated water at the Minami-Kanto gas field in Japan. Geomicrobiol 24:93–100
Narasimhan KS, Mukherjee AK, Sengupta S, Singh SM, Alam MM (1998) Coal bed methane potential in India. Fuel 77:1865–1866
Ogram AV, Jessup RE, Ou LT, Rao PSC (1985) Effects of sorption on biological degradation rates of (2, 4-dichlorophenoxy) acetic acid in soils. Appl Environ Microbiol 49:582–587
Paster BJ, Parola EC (1982) Physiological diversity of rumen Spirochetes. Appl Environ Microbiol 43:686–693
Penner TJ, Foght JM, Budwill K (2010) Microbial diversity of western Canadian subsurface coal beds and methanogenic coal enrichment cultures. Int J Coal Geol 82:81–93
Ramaswami A, Luthy RG (1997) Measuring and modeling physicochemical limitations to bioavailability and biodegradation. In: Hurst CJ (ed) Manual of environmental microbiology. ASM, Washington, DC, pp 721–729
Reilly CO, Colleran E (2005) Toxicity of nitrite toward mesophilic and thermophilic sulphate-reducing, methanogenic and syntrophic populations in anaerobic sludge. J Ind Microbiol Biotechnol 32:46–52
Roy R, Conrad R (1999) Effect of methanogenic precursors (acetate, hydrogen, propionate) on the suspension of methane production by nitrate in anoxic rice field soil. FEMS Microbiol Ecol 28:49–61
Scott AR (1999) Improving coal gas recovery with microbially enhanced coalbed methane. In: Mastalerz M, Glikson M, Golding SD (eds) Coalbed methane: scientific, environmental and economic evaluation. Kluwer, Dordrecht, pp 89–110
Sekiguchi Y, Kagamata Y, Syutsubo K, Ohashi A, Harada H (1998) Phylogenetic diversity of mesophilic and thermophilic granular sludges determined by 16S rRNA gene analysis. Microbiology 144:2655–2665
Shimizu S, Akiyama M, Naganuma T, Fujioka M, Nako M, Ishijima Y (2007) Molecular characterization of microbial communities in deep coal seam groundwater of northern Japan. Geobiology 5:423–433
Singh AK, Sharma M, Singh MP (2009) Genesis of natural cokes: some Indian examples. Int J Coal Geol 75:40–48
Skladany GJ, Baker KH (1994) Laboratory biotreatability studies. In: Baker KH, Herson DS (eds) Bioremediation. McGraw-Hill, New York, pp 97–172
Strąpoc D, Picardal FW, Turich C, Schaperdoth I, Macalady JL, Lipp JS, Lin YS, Ertefai TF, Schubotz F, Hinrichs KU, Mastalerz M, Schimmelmann A (2008) Methane-producing microbial community in a coal bed of the Illinois Basin. Appl Environ Microbiol 74:2424–2432
Thielemann T, Cramer B, Schippers A (2004) Coalbed methane in the Ruhr Basin, Germany: a renewable energy source? Org Geochem 35:1537–1549
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tierney M (2006) Naphthalene degradation by denitrifying bacteria. ProQuest dissertations and theses, Rutgers, The State University of New Jersey, New Brunswick, 164 p. http://search.proquest.com/docview/305291617?accountid=26421
Volkwein JC, Schoeneman AL, Clausen EG, Gaddy JL, Johnson ER, Basu R, Ju N, Klasson KT (1994) Biological production of methane from bituminous coal. Fuel Process Technol 40:339–345
Widdel F (1986) Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors. Appl Environ Microbiol 51:1056–1062
Willmann G, Fakoussa RM (1997) Extracellular oxidative enzymes of coal-attacking fungi. Fuel Process Technol 52:27–41
Winfrey MR, Zeikus JG (1977) Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol 33:275–281
Wolin EA, Wolin MJ, Wolfe RS (1963) Formation of methane by bacterial extracts. J Biol Chem 238:2882–2886
Zhao Y, Zhang H, Boone DR, Mah RA (1986) Isolation and characterization of a fast-growing, thermophilic Methanobacterium species. Appl Environ Microbiol 52:1227–1229
Zinder SH (1993) Physiological ecology of methanogens. In: Ferry JG (ed) Methanogenesis: ecology, physiology, biochemistry and genetics. Chapman and Hall, New York, pp 128–206
Acknowledgements
This work was carried out jointly by School of Biotechnology, Banaras Hindu University and Institute of Reservoir Studies, Ahmedabad with financial support from Oil and Natural gas Commission, New Delhi and Department of Biotechnology, Government of India. DNS was supported by a Junior/Senior Research Fellowship from the University Grants Commission, New Delhi. We appreciate the critical help and advice received from Dr D. M. Kale (ONGC, New Delhi); Dr R. V. Marathe, Dr S. Bateja and Dr T. R. Misra (Institute of Reservoir Studies, Ahmedabad), Dr D. R. Ranade (Agarkar Research Institute, Pune) and Dr S. P. Chowdhury (Helmholtz Zentrum, Muenchen).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00253-012-3879-5.
Rights and permissions
About this article
Cite this article
Singh, D.N., Kumar, A., Sarbhai, M.P. et al. Cultivation-independent analysis of archaeal and bacterial communities of the formation water in an Indian coal bed to enhance biotransformation of coal into methane. Appl Microbiol Biotechnol 93, 1337–1350 (2012). https://doi.org/10.1007/s00253-011-3778-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3778-1