Abstract
Epitope vaccine based on urease of Helicobacter pylori is a promising option for prophylactic and therapeutic vaccination against H. pylori infection. In this study, we constructed an epitope vaccine with mucosal adjuvant cholera toxin B subunit (CTB) and an epitope (UreA183-203) of H. pylori urease A subunit named CTB-UA. The CTB-UA fusion protein was expressed in Escherichia coli, and the purified protein was used for intraperitoneal immunization experiments in BALB/c mice. The experimental results indicated that anti-CTB-UA antibody could recognize both H. pylori urease A subunit (UreA) and urease B subunit (UreB). Besides, the CTB-UA epitope vaccine had good immunogenicity and immunoreactivity and could induce specific neutralizing antibodies which showed effectively inhibitory effect on the enzymatic activity of H. pylori urease. CTB-UA is a promising molecule to be investigated as H. pylori vaccine antigen candidate.
Similar content being viewed by others
Introduction
Helicobacter pylori is a gram-negative spiral bacterium that infects greater than 50% of the world population and can cause a variety of diseases, including chronic gastritis, peptic ulcers, gastric adenocarcinoma, and gastric lymphoma (Labenz and Borsch 1994; Blaser 1990; Uemura et al. 2001). H. pylori has been categorized by World Health Organization as a class I human carcinogen. The current treatment requiring multidrug regimens for H. pylori infection, a combination of at least two antibiotics and a proton-pump inhibitor, has a number of drawbacks including poor patient compliance, increasing antibiotic resistance, side-effects of the antibiotics, re-infection, and high cost (Cheng and Hu 2005; Boyanova et al. 2002; Kwon et al. 2010). Vaccination would be a cost-effective means to control this public health problem faced by one half of the world's population.
H. pylori urease is a polymeric enzyme that comprises two subunits, UreA (29.5 kDa) and UreB (66 kDa). H. pylori can produce large amounts of urease, and a proportion of H. pylori urease becomes associated with the bacterial surface. Urease permits H. pylori to maintain a constant internal and periplasmic pH to protect bacteria from acid. In addition to its role in colonization, urease might participate in tissue damage via the production of ammonia (Marshall et al. 1990; Eaton et al. 1991). The fact that the synthesis of active urease is a complex process involving many different proteins indicates that urease is an important target for prophylactic and therapeutic vaccine development.
Nonetheless, H. pylori urease-specific polyclonal IgG antibodies generated by immunization with purified H. pylori urease protein did not inhibit its enzymatic activity at all (Nagata et al. 1992). This result suggested that there might be two types of urease-specific antibodies; one may help to promote its enzymatic activity and aggravate the gastric disorder, and the other may be beneficial in inhibiting its enzymatic activity and preventing bacterial attachment to the gastric mucosa. Besides, it has recently been reported that poor response to the urease may favor persistence of H. pylori infection (Leal-Herrera et al. 1999). Therefore, it may be favorable to prevent or treat H. pylori-related diseases by epitope vaccine composed of carefully chosen urease epitope which can induce neutralizing antibody.
Several prophylactic and therapeutic vaccines against H. pylori have been developed, including whole-cell vaccine (Raghavan et al. 2002; Nystrom et al. 2006), recombinant subunit antigen vaccine (Rossi et al. 2004), and DNA vaccine, but no major breakthrough has been achieved. Epitope vaccine is a new strategy for inducing a specific immune response against H. pylori infection, avoiding the side effects of other unfavorable epitopes in the complete antigen. It has been reported that several epitope vaccines against H. pylori can afford prophylactic and therapeutic effects against H. pylori infection in BALB/c mice, but they are mainly composed of epitopes from UreB and induce the antibodies specific for UreB (Zhou et al. 2009; Zhao et al. 2007). A mouse monoclonal antibody (mAb), named HpU-2, showed the strongest inhibitory effect on the enzymatic activity of the urease suppressing the urease activity by 82% in 26 urease monoclonal antibodies (Nagata et al. 1992). Further research indicated that the HpU-2 mAbs mainly recognized UreA but weakly UreB and the epitope sequence recognized by HpU-2 mAb was a stretch of UreA-derived 21 amino acid residues (UreA183-203) (Fujii et al. 2004; Hifumi et al. 2006). Therefore, we think an epitope vaccine, which can induce antibodies specific for both UreA and UreB, may be a better candidate for controlling H. pylori infection.
In this study, we constructed an epitope vaccine, designated CTB-UA, composed of the mucosal adjuvant CTB and a B cell epitope from UreA (UreA183-203). The CTB-UA fusion protein was expressed in Escherichia coli in an active pentameric form, and its immunogenicity and immunoreactivity was evaluated by intraperitoneal immunization experiments in BALB/c mice.
Materials and methods
Bacterial strains and plasmids
The recombinant plasmid pET-CtUBE for highly expressing CtUBE was constructed by inserting the recombinant gene into NcoI/XhoI sites of the expression vector pET28a (Novagen, USA) under the control of the T7 promoter (Zhao et al. 2007). E. coli DH5α (ATCC 53868) was used as the host for propagating plasmids. E. coli BL21 (DE3), containing the T7 RNA polymerase gene under the control of the lac promoter in a lysogenic form, was used for expressing the fusion proteins.
Epitope vaccine design and plasmid construction
The theoretically optimal combination of the intra-molecule adjuvant CTB, UreA183-203, and the linker between CTB and UreA183-203 for the epitope vaccine was established on the basis of modeling and prediction using RANKPEP and DNA star software. The linker between CTB and UreA183-203 was designed to be a seven-amino-acid, proline-containing segment (DPRVPSS) (Clements 1990). The fusion protein CTB-UA expression plasmid pETCUA was constructed by subcloning the ctb gene and ureA 183-203 gene into pET22b (Novagen, USA). Two complementary single-stranded DNA (ssDNA) sequences coding ureA 183-203 gene were synthesized: P1-ureA 183-203 (5′-CGAGCAGCAGCGTGGAACTGATTGATATTGGCGGCAACCGCCGCATTTTTGGCTTTAACGCGCTGGTGGATC-3′), containing a KpnI restriction site and P2-ureA 183-203 (5′-TCGAGATCCACCAGCGC GTTAAAGCCAAAAATGCGGCGGTTGCCGCCAATATCAATCAGTTCCACGCTGCTGCTCGGTAC-3′), containing a XhoI restriction site.
Fusion protein expression and purification
The fusion protein CTB-UA was expressed and purified according to the protocol performed as previous described (Zhao et al. 2007) with some modification. The recombinant vector pETCUA was transformed into E. coli BL21 (DE3) for expression of the recombinant protein CTB-UA. After induction with IPTG, bacteria were harvested by centrifugation. The pellets were lysed by the addition of a 0.2-fold volume of lysis solution at 37°C for 30 min. Then, the bacterial lysates were disrupted by mild sonication at 4°C for protein pattern analysis in soluble and insoluble cell fractions. The recombinant protein CTB-UA was purified by Ni2+-charged column chromatography (Bio Basic Inc, Markham, Canada) according to the recommendation of the manufacturer. The obtained fusion proteins were dialyzed in 2 l of 30 mM Tris–HCl buffer (pH 8.0) in one step sufficiently. Finally, the recombinant proteins were then purified by anion-exchange chromatography using DEAE Sepharose FF (Amersham Pharmacia Biotech AB, Sweden) in binding buffer and eluted with elution buffer. After purification, the purity of the fusion peptide CTB-UA was analyzed by 12% SDS-PAGE and computer scan. The samples were dialyzed in 2 l of PBS and finally concentrated and stored at −70°C.
SDS-PAGE of the CTB-UA fusion protein and GM1-ELISA
In order to analyze the ability of the CTB-UA fusion protein to fold into pentamers, the samples of CTB-UA fusion protein were not boiled and a sample buffer that did not contain β-mercaptoethanol was used in12% SDS-PAGE. The ability of the pentamers to bind to its cellular receptor was assessed by GM1 ganglioside enzyme-linked immunosorbent assay (GM1-ELISA). The experimental procedure was performed as previously described (Menezes et al. 2002). Briefly, ELISA plates (Corning Costar Corp, MA, USA) were coated with GM1 ganglioside (Sigma, St. Louis, USA) or bovine serum albumin (10 μg/ml, 100 μl/well) at 4°C overnight and blocked with 5% bovine serum albumin (BSA) for 2 h at 37°C. After washing three times with PBS solution containing 0.05% Tween-20 (PBST), the recombinant proteins were diluted, from 100 to 0.78 μg/ml in PBST, added to the plates, and incubated for 1 h at 37°C. Unbound proteins were removed from the plate by washing three times with PBST, and then, a proper dilution of anti-CTB polyclonal antibody (Biomade technology, Qingdao, China) was added to the plates and incubated for 1 h at 37°C. After washing, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (Jackson ImmunoResearch, USA) was added, and the plate was incubated at 37°C for 1 h. The color reaction based on Tetramethylbenzidine (TMB, TianGen Biotech, Beijing, China) was terminated after incubation for 10 min at room temperature by the addition of 50 μl of H2SO4 (2 M), and the absorbance at 450 nm was measured by microplate reader.
Immunization and sample collection
Specific pathogen-free (SPF) male BALB/c mice or SD rats, 5 to 6 weeks old, were purchased from Comparative Medicine Center of Yangzhou University. All animal experiments were approved by the Animal Ethical and Experimental Committee of China Pharmaceutical University.
SPF BALB/c mice or SD rats were randomized into four groups (five mice or rats in each group) and were respectively immunized with 100 or 200 μg of the purified CTB-UA, recombinant cholera toxin B subunit (rCTB), recombinant UreB protein (rUreB), and PBS. The fusion proteins in PBS were emulsified with an equal volume of complete Freund′s adjuvant (Sigma, St. Louis, USA) for the first vaccination and with incomplete Freund adjuvant (Sigma, St. Louis, USA) for the second and third vaccinations. The fusion proteins in PBS without adjuvant were for the last booster vaccination. Antisera were separated on the fifth day after the last booster.
Western blot
Purified recombinant protein CTB-UA, rCTB, recombinant UreA protein (rUreA, Linc-Bio, Shanghai, China), and rUreB were applied to 12% SDS-PAGE under denaturing conditions and electro-transferred on to a nitrocellulose membrane at 80 mA for 2 h. Nonspecific binding sites were blocked overnight at 4°C in blocking buffer. Membranes were washed three times for 10 min with PBST and further incubated with a 1:1,000 dilution of mouse polyclonal anti-CTB-UA serum, anti-rCTB serum or normal mouse serum at 37°C for 1 h. After being washed three times with PBST, the membrane was incubated in HRP-conjugated goat anti-mouse IgG (1:10,000) at 37°C for 2 h. The protein bands were visualized with enhanced chemiluminescence detection system (Amersham, UK).
Assessment of antigen-specific antibody responses
Antigen-specific antibodies were measured by an ELISA assay. Briefly, ELISA plates were coated with 0.5 μg/well of natural H. pylori urease (Linc-Bio, Shanghai, China), rUreA, or rUreB at 4°C overnight. The plates were washed with PBST, and blocked with 5% (w/v) BSA in PBS. The plates were then washed with PBST and incubated with 100 μl of mouse or rat sera, serially diluted in PBS at 37°C for 1 h. After washing, HRP-conjugated goat anti-mouse IgG or HRP-conjugated goat anti-rat IgG (General Bioscience Corporation, USA) was added, and the plates were incubated again for 1 h. The color reaction based on TMB was terminated after incubation for 10 min at room temperature by the addition of 50 μl of H2SO4 (2 M), and the absorbance at 450 nm was measured by microplate reader. Serum samples from mice and rats were assayed in triplicate.
Assessment of epitope peptide-specific antibody response
The epitope peptides of SVELIDIGGNRRIFGFNALVD (UreA183-203) were synthesized commercially (TASH, Shanghai, China) by the Fmoc solid-phase method. The synthesized peptides were purified and analyzed by reverse-phase HPLC, and then, the purified peptides were identified by use of a mass spectrometer. The purity of the epitope peptide was 95.27%. The peptides were coated overnight on an ELISA plate at 4°C (10 μg/ml, 100 μl/well) and the subsequent steps of the assay were performed as described above for the indirect ELISA. All assays were performed in triplicate.
Urease inhibition assay by fusion protein-specific polyclonal antibody
A urease neutralization test was performed as previously described (Fujii et al. 2004). Briefly, the purified natural H. pylori urease (2 μg in 50 μl) was pre-incubated with 50 μl serial dilutions of antiserum from different groups in 96-well microtiter plates overnight at 4°C. After pre-incubation, 100 μl of the above reaction solution was mixed with 100 μl of 50 mM phosphate buffer (pH 6.8) containing 500 mM urea, 0.02% phenol red, and 0.1 mM dithiothreitol (DTT). The plates were incubated at 37°C. Color development was measured at 550 nm at 30 min intervals over a period of 3 h. Percentage inhibition was determined by the following equation:
Statistical analyses
All the statistical analyses were performed with the GraphPad Prism 5 software. Data were expressed as mean ± standard deviation (S.D). Statistical significance was tested using Student's paired t test. p < 0.05 was considered as statistically significant. *p < 0.05, **p < 0.01, and ***p < 0.001 are considered as not significant.
Results
Design of epitope vaccine
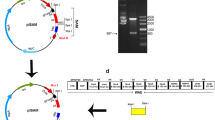
In order to retain the immunological function of the UreA epitope (UreA183-203) and further enhance the immunogenicity of UreA183-203, we constructed a CTB-UA fusion protein with scientific and reasonable structure on the basis of prediction and modeling using RANKPEP and DNAstar software. The protein structure of the epitope vaccine CTB-UA is shown in Fig. 1. The UreA epitope (UreA183-203) was designed to fuse with the C-terminal of CTB and a seven-amino-acid, proline-containing segment (DPRVPSS) was used as a spacer at linkage sites between CTB and UreA183-203 to decrease the interaction between them. The construction process of recombinant plasmid pETCUA containing CTB-UA fusion gene is shown in Fig. 2.
Schematic representation of the designed epitope vaccine CTB-UA. CTB was selected as the intra-mucosal adjuvant to increase the immunogenic activity of the B cell epitope UreA183-203. In order to retain the immune function of the B cell epitope UreA183-203, CTB and UreA183-203 were linked with the linker (DPRVPSS) to avoid the formation of new epitopes
Construction of the recombinant plasmid pETCUA. The recombinant plasmid pETCUA containing CTB-UA fusion gene was constructed by subcloning the ctb gene and ureA 183-203 gene into pET22b. The ctb gene was cloned by PCR amplification from pET-CtUBE. The ctb gene was cloned into pET22b through NcoI and HindIII restriction sites, generating the plasmid pETC. Two complementary single-stranded DNA (ssDNA) sequences coding ureA 183-203 gene were synthesized, mixed together, annealed, and inserted subsequently into the plasmid pETC through KpnI and XhoI restriction sites, generating the recombinant plasmid pETCUA
Soluble expression and purification of CTB-UA
The recombinant protein CTB-UA was expressed in E. coli BL21 (DE3) and identified by its molecular mass (16 kDa). The results showed that the fusion protein CTB-UA was mainly expressed in soluble form (Fig. 3a) and had a high level of expression (about 23% of total bacterial protein). After purification by Ni2+-charged column chromatography and anion-exchange chromatography, the purity of the fusion protein CTB-UA, analyzed by 12% SDS-PAGE (Fig. 3b) and computer scan, was 94.8%.
The soluble expression and purification of CTB-UA analyzed by SDS-PAGE. a The soluble expression of CTB-UA was analyzed by 12% SDS-PAGE. Lane 1: the whole bacterial proteins of E. coli BL21 (DE3) expressing CTB-UA (16 kDa). Lane 2: the soluble proteins of E. coli BL21 (DE3) expressing CTB-UA (16 kDa). b Analysis of purified CTB-UA by 12% SDS-PAGE. Lane 1: CTB-UA purified by Ni2+-charged column chromatography; lane 2: CTB-UA purified by DEAE sepharose FF chromatography
Pentamer formation and GM1-ELISA
In order to analyze the ability of CTB-UA to form pentamers, the purified fusion protein CTB-UA was resuspended in sample buffer without β-mercaptoethanol and was directly loaded onto a 12% SDS-PAGE (Fig. 4a). In the reduced and boiled samples, a single band of 16 kDa (Fig. 4a, lane 1) was observed because CTB-UA could not form pentamers. However, in the non-reduced and non-boiled samples, a band of about 80 kDa (Fig. 4a, lane 2), the predicted size of the pentameric form of CTB-UA, was detected.
Analysis of CTB-UA pentamer formation and its ability to bind GM1 receptor. a The fusion protein CTB-UA was loaded onto a 12% SDS-PAGE to evaluate its pentamer formation. Lane 1: The sample was boiled in reducing conditions. CTB-UA could not form pentamers and a single band of 16 kDa was observed. Lane 2: the sample was not submitted to boiling in non-reducing conditions. A band of about 80 kDa can be observed in non-boiled and non-reduced samples. b A GM1-ELISA was performed to confirm the ability of the CTB-UA pentamers to bind GM1. The rCTB was used as a positive control. The ELISA was performed by coating a 96-well plate with GM1 or BSA
A GM1-ELISA was performed to confirm the ability of the CTB-UA pentamers to bind GM1. We used rCTB that was able to bind GM1 as positive control. Besides, as negative control, CTB-UA and rCTB were evaluated using BSA as the coating protein. CTB-UA and rCTB were able to bind GM1 in a dose-dependent manner (Fig. 4b). In addition to this, their curves presented the same profile. These experimental results confirmed that CTB-UA had the ability to form pentamers, and the presence of five molecules of UreA epitope peptides didn't abrogate the binding of the pentamers to its receptor.
Assessment of fusion protein-immunized mouse sera by Western blot
From a qualitative point of view, the immunogenicity and immunoreactivity of the CTB-UA fusion protein was assessed by Western blotting. Mouse anti-CTB-UA serum could react with CTB-UA, rCTB, rUreA, and rUreB specifically (Fig. 5a), while normal mouse serum did not react with any one (Fig. 5c). The results showed that the fusion protein CTB-UA had the immunological function of the UreA epitope and could induce specific antibodies against H. pylori urease. Moreover, mouse anti-rCTB serum could react with CTB-UA (Fig. 5b), suggesting that the CTB component was present in the fusion protein and the fusion protein did not undergo degradation.
Western blotting of recombinant epitope vaccine. a The fusion protein CTB-UA, rCTB, rUreA, and rUreB were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Mouse anti-CTB-UA serum was used for detecting immunoreactivity to rUreA and rUreB. b Mouse anti-CTB serum was used for detecting CTB component in CTB-UA. c Normal mouse serum was used as control
Assessment of fusion protein-immunized mouse sera by ELISA
From a quantitative point of view, the immunogenicity and immunoreactivity of the CTB-UA fusion protein was assessed by ELISA. After immunization, serum was collected from each mouse and antigen-specific antibody levels against three antigens (natural H. pylori urease, rUreA, and rUreB) were measured by ELISA. A modest antibody level was observed in sera from mice immunized with the fusion protein CTB-UA. Compared with intraperitoneal immunization with rCTB or PBS, intraperitoneal immunization with CTB-UA significantly increased the levels of specific IgG (p < 0.001) against natural H. pylori urease, rUreA, or rUreB (Fig. 6). There was no significant difference between CTB-UA-immunized group and rUreB-immunized group on the level of specific antibodies against natural H. pylori urease, indicating that the fusion protein CTB-UA had good immunogenicity and immunoreactivity. In addition, the same results were obtained from the rats immunized with CTB-UA (Fig. 7), except that the specific antibody level of CTB-UA-immunized rats were lower than that of CTB-UA-immunized mice.
Detection of specific antibodies in CTB-UA-immunized mice by ELISA. Data are expressed as mean ± S.D. The mice were immunized with CTB-UA, rCTB, rUreB, or PBS by intraperitoneal injection. Antisera samples were collected on the fifth day after the last booster. The serum was diluted 1:5,000. p < 0.05 was considered as statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant
Detection of specific antibodies in CTB-UA-immunized rats by ELISA. Data are expressed as mean ± S.D. The rats were immunized with CTB-UA, rCTB, rUreB, or PBS by intraperitoneal injection. Antisera samples were collected on the fifth day after the third booster. The serum was diluted 1:2,000. p < 0.05 was considered as statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ns not significant
To examine the specific anti-peptide antibody levels elicited by the CTB-UA, the anti-UreA183-203 antibody response was evaluated by synthesizing the UreA183-203 peptide.
Intraperitoneal immunization with CTB-UA in both mice and rats significantly increased the levels of specific IgG (p < 0.001) against the UreA183-203 peptide compared with intraperitoneal immunization with rCTB, rUreB, or PBS (Figs. 6 and 7). The results indicated that anti-urease antibodies induced by CTB-UA were mainly anti-UreA183-203 antibodies different from that triggered by rUreB.
Inhibition of urease activity by fusion protein-specific polyclonal antibody
In order to test the effect of antibodies induced by CTB-UA, a urease neutralization assay was performed. The purified natural H. pylori urease was pre-incubated with a serial dilution of IgG from mice immunized with CTB-UA, rUreB, or rCTB, and the inhibitory effect of the antibodies on the enzymatic activity of H. pylori urease was assayed. The inhibition by anti-CTB-UA polyclonal antibodies or anti-rUreB polyclonal antibodies was dose-dependent and the former is more excellent. However, anti-rCTB polyclonal antibodies didn't inhibit the enzymatic activity of H. pylori urease obviously (Fig. 8). This result indicated that the antibodies induced by CTB-UA have neutralization activity and the ability of anti-CTB-UA polyclonal antibodies to inhibit the urease activity was better than that of anti-rUreB polyclonal antibodies, which may be due to the presence of neutralizing antibodies against H. pylori urease with a higher specificity induced by the UreA183-203 epitope within CTB-UA.
Inhibition of H. pylori urease activity by fusion protein-specific antibody. The purified natural H. pylori urease was pre-incubated with a serial dilution of IgG from mice immunized with CTB-UA, rUreB, or rCTB. The optical density of the mixture was determined at 550 nm by the indicator of phenol red. The data are expressed as percentage inhibition
Discussion
H. pylori infection is one of the most common infections in human beings worldwide. It causes chronic gastritis, peptic ulcers, and is an important risk factor for gastric cancer later in life. H. pylori infections cause very high morbidity and mortality, and they impose a major burden upon health care systems worldwide. Vaccination against H. pylori, both to prevent and to treat H. pylori infection, is an attractive strategy, either as an alternative or a complementary to antibiotic treatment. H. pylori urease is an important target for prophylactic and therapeutic vaccine development for its outstanding features. Several preventive and therapeutic vaccines based on H. pylori urease have been developed, being mainly genetically engineered subunit vaccines (Londono-Arcila et al. 2002; DiPetrillo et al. 1999; Wehrle et al. 1963; Bumann et al. 2001), but no major breakthrough has been achieved. Epitope vaccine can induce a specific immune response against H. pylori infection and had a much better specificity and security than other vaccines. Consequently, we think that an epitope vaccine, particularly based on H. pylori urease, may be a potential candidate for controlling H. pylori infection.
The design of an epitope vaccine is very important, and a number of factors have been shown to influence its overall success at inducing an immune response against the desired peptide sequence, and more importantly, to induce neutralizing antibodies against the pathogen (Liu et al. 2005; Liu et al. 2004; Yano et al. 2005). Epitopes have very low immunogenicity for their low molecular weight. How to enhance the immunogenicity of small molecule peptides is a hot and difficult problem. In order to elicit an effective immune response, it is a common practice to couple epitopes to a carrier protein. In this study, we constructed an epitope vaccine named CTB-UA composed of the mucosal adjuvant CTB and a B cell epitope from UreA. In order to decrease the interaction between CTB and UreA183-203, a seven-amino-acid, proline-containing segment (DPRVPSS) was used as a spacer at linkage sites between them. It has been proven that the linker (DPRVPSS) could retain the antigenicity of E. coli heat stable enterotoxin (ST) in the LTB-ST fusion protein (Clements 1990). ST consisting of 19 amino acid residues was similar to an epitope, so we still chose the linker (DPRVPSS) to decrease the interaction between CTB and UreA183-203 in constructing the CTB-UA epitope vaccine. Besides, DNAstar software predicted that there was no generation of new epitopes at linkage sites between CTB and UreA183-203. Our results showed that the CTB-UA epitope vaccine had good immunogenicity and immunoreactivity in BALB/c mice and SD rats.
CTB is a potent mucosal adjuvant and chemical conjugations with CTB have been performed using many different heterologous antigens (Sun et al. 1999; Kang et al. 2003). The pentameric form of CTB is responsible for the binding to the GM1 ganglioside, which is present on all nucleated mammalian cells and abundant on intestinal epithelial cells. The mucosal carrier and associated immunological properties of CTB are thought to be critically dependent on its pentameric structure and its ability to bind to GM1 receptors. These features facilitate the uptake of coupled antigens across the mucosal barrier and have also been found to lead to a greatly enhanced presentation of carried antigens by all antigen-presenting cells tested. The experimental results confirmed that CTB-UA fusion protein had the ability to form pentamers, and the presence of five molecules of UreA epitope peptides didn't abrogate the binding of the pentamers to its receptor.
To examine the immunogenicity and immunoreactivity of the CTB-UA epitope vaccine, many antigens were referred, including natural H. pylori urease, rUreA, and rUreB. Because natural H. pylori urease didn't contain protein impurities from E. coli host, it was more truthful and accurate to detect the level of specific antibody against H. pylori using natural H. pylori urease than recombined urease. The results showed that the antibodies induced by the CTB-UA epitope vaccine could react with those antigens specifically and there was no significant difference between CTB-UA-immunized group and rUreB-immunized group on the level of specific antibodies against natural H. pylori urease. Besides, the UreA183-203 peptide was synthesized and used to examine the peptide-specific antibody response. The results indicated that the CTB-UA epitope vaccine was capable of generating antibodies directed specifically against the UreA183-203 region of UreA.
Previous studies have found that some monoclonal antibodies (mAbs) against H. pylori urease have the ability to inhibit enzymatic activity (Qiu et al. 2010; Fujii et al. 2004; Hirota et al. 2001), whereas urease-specific polyclonal antibodies generated by immunization with purified H. pylori urease did not inhibit its enzymatic activity at all. Therefore, it may be favorable to prevent or treat H. pylori-related diseases by epitope vaccine composed of carefully chosen urease epitope which can induce neutralizing antibody. UreB is considered a potential antigen for the development of prophylactic and therapeutic vaccines against H. pylori infection, whereas there are more and more evidences suggesting that UreA is likewise an important target for prophylactic and therapeutic vaccine development (Hifumi et al. 2006; Lucas et al. 2001; Gomez-Duarte et al. 1998). The UreA183-203 is a B cell epitope which is recognized by HpU-2 monoclonal antibody (mAb). It is interesting that the HpU-2 mAb can recognize both UreA and UreB, by which UreA is recognized more strongly than UreB. Moreover, HpU-2 showed the strongest inhibitory effect on the enzymatic activity of the urease suppressing the urease activity by 82% (Fujii et al. 2004). The results indicated that the antibodies induced by CTB-UA have neutralization activity and the ability of anti-CTB-UA polyclonal antibodies to inhibit the urease activity was better than that of anti-rUreB polyclonal antibodies, which may be due to the presence of urease neutralizing antibodies with a higher specificity induced by the UreA183-203 epitope within CTB-UA. Compared with the prophylactic or therapeutic epitope vaccine against H. pylori published before (Zhou et al. 2009; Zhao et al. 2007), the epitope vaccine CTB-UA exhibits several advantages. Firstly, the fusion protein CTB-UA is expressed in E. coli as a soluble protein, reducing protein loss during purification and renaturation. Secondly, the epitope vaccine CTB-UA can produce high levels of anti-urease antibodies compared to UreB, indicating it has good immunogenicity. Thirdly, the specific antibodies induced by CTB-UA can recognize both UreA and UreB and belong to neutralizing antibodies against H. pylori urease.
In conclusion, we have designed and constructed an epitope vaccine CTB-UA with good immunogenicity and immunoreactivity, which could induce specific neutralizing antibodies against UreA and UreB. Ongoing studies will evaluate the prophylactic and therapeutic effects of the epitope vaccine CTB-UA against H. pylori infection in BALB/c mice. This study will provide much experimental evidences for the development of epitope vaccines for human use.
References
Blaser MJ (1990) Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis 161(4):626–633
Boyanova L, Mentis A, Gubina M, Rozynek E, Gosciniak G, Kalenic S, Goral V, Kupcinskas L, Kantarceken B, Aydin A, Archimandritis A, Dzierzanowska D, Vcev A, Ivanova K, Marina M, Mitov I, Petrov P, Ozden A, Popova M (2002) The status of antimicrobial resistance of Helicobacter pylori in eastern Europe. Clin Microbiol Infect 8(7):388–396
Bumann D, Metzger WG, Mansouri E, Palme O, Wendland M, Hurwitz R, Haas G, Aebischer T, von Specht BU, Meyer TF (2001) Safety and immunogenicity of live recombinant Salmonella enterica serovar Typhi Ty21a expressing urease A and B from Helicobacter pylori in human volunteers. Vaccine 20(5–6):845–852
Cheng H, Hu FL (2005) The epidemiology of Helicobacter pylori resistance to antibiotics in Beijing. Zhonghua Yi Xue Za Zhi 85(39):2754–2757
Clements JD (1990) Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect Immun 58(5):1159–1166
DiPetrillo MD, Tibbetts T, Kleanthous H, Killeen KP, Hohmann EL (1999) Safety and immunogenicity of phoP/phoQ-deleted Salmonella typhi expressing Helicobacter pylori urease in adult volunteers. Vaccine 18(5–6):449–459
Eaton KA, Brooks CL, Morgan DR, Krakowka S (1991) Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun 59(7):2470–2475
Fujii R, Morihara F, Oku T, Hifumi E, Uda T (2004) Epitope mapping and features of the epitope for monoclonal antibodies inhibiting enzymatic activity of Helicobacter pylori urease. Biotechnol Bioeng 86(4):434–444
Gomez-Duarte OG, Lucas B, Yan ZX, Panthel K, Haas R, Meyer TF (1998) Protection of mice against gastric colonization by Helicobacter pylori by single oral dose immunization with attenuated Salmonella typhimurium producing urease subunits A and B. Vaccine 16(5):460–471
Hifumi E, Yamada Y, Uda T (2006) A catalytic antibody heavy chain HpU-2 degrading its epitope peptide and H. pylori urease. Immunol Lett 103(1):68–74
Hirota K, Nagata K, Norose Y, Futagami S, Nakagawa Y, Senpuku H, Kobayashi M, Takahashi H (2001) Identification of an antigenic epitope in Helicobacter pylori urease that induces neutralizing antibody production. Infect Immun 69(11):6597–6603
Kang SM, Yao Q, Guo L, Compans RW (2003) Mucosal immunization with virus-like particles of simian immunodeficiency virus conjugated with cholera toxin subunit B. J Virol 77(18):9823–9830
Kwon SB, Lee KL, Kim JS, Lee JK, Kim W, Jung YJ, Jeong JB, Kim JW, Kim BG (2010) Antibiotics-associated diarrhea and other gastrointestinal abnormal responses regarding Helicobacter pylori eradication. Korean J Gastroenterol 56(4):229–235
Labenz J, Borsch G (1994) Evidence for the essential role of Helicobacter pylori in gastric ulcer disease. Gut 35(1):19–22
Leal-Herrera Y, Torres J, Perez-Perez G, Gomez A, Monath T, Tapia-Conyer R, Munoz O (1999) Serologic IgG response to urease in Helicobacter pylori-infected persons from Mexico. Am J Trop Med Hyg 60(4):587–592
Liu W, Peng Z, Liu Z, Lu Y, Ding J, Chen YH (2004) High epitope density in a single recombinant protein molecule of the extracellular domain of influenza A virus M2 protein significantly enhances protective immunity. Vaccine 23(3):366–371
Liu Z, Wang Z, Chen YH (2005) Predefined spacers between epitopes on a recombinant epitope-peptide impacted epitope-specific antibody response. Immunol Lett 97(1):41–45
Londono-Arcila P, Freeman D, Kleanthous H, O'Dowd AM, Lewis S, Turner AK, Rees EL, Tibbitts TJ, Greenwood J, Monath TP, Darsley MJ (2002) Attenuated Salmonella enterica serovar Typhi expressing urease effectively immunizes mice against Helicobacter pylori challenge as part of a heterologous mucosal priming-parenteral boosting vaccination regimen. Infect Immun 70(9):5096–5106
Lucas B, Bumann D, Walduck A, Koesling J, Develioglu L, Meyer TF, Aebischer T (2001) Adoptive transfer of CD4+ T cells specific for subunit A of Helicobacter pylori urease reduces H. pylori stomach colonization in mice in the absence of interleukin-4 (IL-4)/IL-13 receptor signaling. Infect Immun 69(3):1714–1721
Marshall BJ, Barrett LJ, Prakash C, McCallum RW, Guerrant RL (1990) Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology 99(3):697–702
Menezes CA, Amianti J, Harayama HS, Koga PC, Trabulsi LR, Piazza RM (2002) Inhibition of Escherichia coli heat-labile enterotoxin by neoglycoprotein and anti-lectin antibodies which mimic GM1 receptor. FEMS Microbiol Lett 216(1):67–70
Nagata K, Mizuta T, Tonokatu Y, Fukuda Y, Okamura H, Hayashi T, Shimoyama T, Tamura T (1992) Monoclonal antibodies against the native urease of Helicobacter pylori: synergistic inhibition of urease activity by monoclonal antibody combinations. Infect Immun 60(11):4826–4831
Nystrom J, Raghavan S, Svennerholm AM (2006) Mucosal immune responses are related to reduction of bacterial colonization in the stomach after therapeutic Helicobacter pylori immunization in mice. Microbes Infect 8(2):442–449
Qiu Y, Wang YC, Tao HX, Zhan DW, Yuan SL, Wang P, Wang LC, Han XP, Li CS, Li JK, Liu CJ (2010) Identification of B-cell epitopes in urease B subunit of Helicobacter pylori bound by neutralizing antibodies. Vaccine 28(32):5220–5227
Raghavan S, Svennerholm AM, Holmgren J (2002) Effects of oral vaccination and immunomodulation by cholera toxin on experimental Helicobacter pylori infection, reinfection, and gastritis. Infect Immun 70(8):4621–4627
Rossi G, Ruggiero P, Peppoloni S, Pancotto L, Fortuna D, Lauretti L, Volpini G, Mancianti S, Corazza M, Taccini E, Di Pisa F, Rappuoli R, Del Giudice G (2004) Therapeutic vaccination against Helicobacter pylori in the beagle dog experimental model: safety, immunogenicity, and efficacy. Infect Immun 72(6):3252–3259
Sun JB, Mielcarek N, Lakew M, Grzych JM, Capron A, Holmgren J, Czerkinsky C (1999) Intranasal administration of a Schistosoma mansoni glutathione S-transferase-cholera toxoid conjugate vaccine evokes antiparasitic and antipathological immunity in mice. J Immunol 163(2):1045–1052
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345(11):784–789
Wehrle PF, Leedom JM, Portnoy B, Pierce NF, Cowper HH (1963) Safety of sabin oral poliovaccine strains; mass immunization program in Los Angeles County, 1962–1963. JAMA 186:821–826
Yano A, Onozuka A, Asahi-Ozaki Y, Imai S, Hanada N, Miwa Y, Nisizawa T (2005) An ingenious design for peptide vaccines. Vaccine 23(17–18):2322–2326
Zhao W, Wu W, Xu X (2007) Oral vaccination with liposome-encapsulated recombinant fusion peptide of urease B epitope and cholera toxin B subunit affords prophylactic and therapeutic effects against H. pylori infection in BALB/c mice. Vaccine 25(44):7664–7673
Zhou WY, Shi Y, Wu C, Zhang WJ, Mao XH, Guo G, Li HX, Zou QM (2009) Therapeutic efficacy of a multi-epitope vaccine against Helicobacter pylori infection in BALB/c mice model. Vaccine 27(36):5013–5019
Acknowledgments
This work was supported by the Science Foundation of China Pharmaceutical University (grant no, JKY2009023) and Postgraduate Innovation Project of Jiangsu Province (grant no, CXZZ11_0817). We thank especially Professor Wutong Wu for his contributions in implementation of this experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, L., Li, X., Tang, F. et al. Immunological features and the ability of inhibitory effects on enzymatic activity of an epitope vaccine composed of cholera toxin B subunit and B cell epitope from Helicobacter pylori urease A subunit. Appl Microbiol Biotechnol 93, 1937–1945 (2012). https://doi.org/10.1007/s00253-011-3726-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3726-0