Abstract
Type B influenza virus is one of the major epidemic strains and responsible for considerable mortality and morbidity. Rapidly and accurately identifying different influenza B virus lineages, i.e., B/Yamagata (B/Y) and B/Victoria (B/V), is desirable during the flu season. However, the available rapid techniques lack sensitivity, and the usual methods for identifying influenza viruses require expansion of virus in tissue culture or embryonated hen’s eggs. Thus, we developed several sets of primer pairs that were able to detect and distinguish B/Y and B/V in a single real-time PCR assay. Used in conjunction with two sets of specific primers that exhibited purine at 3′ end of at least one primer targeting on HA gene of B/Y and B/V lineages allows us to accurately identify approximately 102 copies per microliter for B/Y and B/V with intra- and inter-assay coefficient of variation (CV) <4%. When it was used to test 17,765 throat swab specimens obtained in the 2006–2010 influenza surveillance season, this method was comparable to hemagglutination inhibition assay in detection, typing and subtyping of influenza viruses with 100% true-negative (specificity) and 100% true-positive (sensitivity). Taken together, this method provides sensitive and robust tool for routine diagnosis and on-time epidemiological examination for WHO decisions on vaccine composition.
Similar content being viewed by others
Introduction
Influenza caused by the influenza virus is an important public health problem with a significant social and economic impact on both industrial and developing countries. It is estimated that influenza can lead to 250,000–500,000 deaths annually worldwide (Stohr 2002), and influenza-associated hospitalizations in the USA range from approximately 54,000 to 430,000 per season with 36,000 deaths (http://www.cdc.gov/flu/about/disease.htm). Historically, four influenza pandemics occurred in humans. The first was the “Spanish Flu” caused by the H1N1 virus that killed 25–50 million people worldwide in 1918; the second was the “Asian Flu” cause by the H2N2 virus that killed 1–4 million people in 1957; the third was the “Hong Kong Flu” cause by the H3N2 virus that killed 0.75–2 million people in 1968. Most recently in 2009, a novel triple-reassortant swine influenza A(H1N1) virus containing a combination of gene segments originated from the avian influenza virus genetic lineage, classical swine genetic lineage, Eurasian swine genetic lineage, and human genetic lineage caused the first influenza pandemic of the twenty-first century reaching an alert level of six raised by the World Health Organization (WHO) (Muhammed Babakir et al. 2009; Garten et al. 2009; Gavin et al. 2009; Carl Kingsford et al. 2009).
Influenza virus are members of the family Orthomyxoviridae with genomes consisting of seven or eight single-stranded negative RNA segments coding for the PB2, PB1, PA, HA, NP, NA, M, and NS genes, respectively. Based on differences in the nucleoprotein and matrix protein, the influenza virus has been divided into three types: A, B, and C. Influenza A virus can be further divided into 16 different HA subtypes (H1–H16) and nine different NA subtypes (N1–N9) based on the difference of two proteins on the surface of the virus: hemagglutinin (HA) and neuraminidase (NA). Influenza B and C viruses each consist of only a single type (Fouchier et al. 2005). Influenza A and B viruses can cause up to severe respiratory disease, with influenza B virus usually existing at low prevalence and causing less severe disease than influenza A virus especially of the H3N2 subtype, while influenza C virus generally causes only mild respiratory illness. Currently, influenza virus A/H1N1 (including novel H1N1 virus), A/H3N2, and B virus are responsible for seasonal influenza epidemics and constitute the components for influenza vaccine (Holmes et al. 2005; Petric et al. 2006; WHO 2011)
Unlike influenza A virus, which contains 16 HA and 9 NA subtypes and often leads to recurrent epidemics and even pandemics on account of its high antigenic change from either gene drift or gene shift, Influenza B virus in current circulation can be divided into two antigenically and genetically distinct lineages referred to as the Yamagata and Victoria lineages designated after their original isolates B/Yamagata/16/88 and B/Victoria/2/87 (Kanegae et al. 1990; Rota et al. 1990). HA protein sequences within each of the two lineages are more than 97% identical, with sequence identity in inter-lineage comparisons at 88–90% on average. Estimates of rate of nucleotide substitutions for influenza B virus ranged from 0.14 × 10−3 to 3.32 × 10−3 substitutions per site per year and 2.68 × 10−3 to 12.50 × 10−3 for influenza A virus (Nelson et al. 2006; Chen and Edward 2008). Influenza B virus exhibits greater stability in both antigenicity and evolutionary dynamics than influenza A virus.
Owing to the capability of frequent antigenic variations of influenza A virus and the circulation of influenza B virus in two lineages, influenza will continue to be an ongoing epidemic disease and a potential pandemic threat to humans. Up to now, vaccination is the only effective method for influenza prevention and control, which depends on the provision of appropriate influenza strains by the international surveillance network. The rapid and accurate determination of annually prevailing influenza strains is therefore the key premise for a quick response to influenza. At present, real-time PCR furnishes a rapid, specific, and sensitive diagnostic method with reduced carryover contamination and applicable to large-scale diagnostics. Many diagnostic real-time PCR methods are available for influenza A virus typing, subtyping, and influenza B typing (Joanna et al. 2007; Suwannakarn et al. 2008; Van et al. 2001; Wu et al. 2008; Shisong et al. 2011). However, real-time PCR methods differentiating the influenza B virus Yamagata and Victoria lineages are relatively scarce other than a report using a unique Taqman MGB probe (Barbara Biere et al. 2010).
To enable quick response to the screening of circulating influenza B virus Yamagata and Victoria lineages, we here developed in the present study a real-time PCR method using traditional Taqman probe based on a single nucleotide mismatch at the end of 3′ primers targeted on the HA gene to the rapid and accurate identification of the influenza B virus lineages in less than 3 h from sample collection to result report.
Materials and methods
Clinical specimens
Throat swab specimens were obtained from out-patients with symptoms of influenza-like illness (ILI) in 2006 to 2010. The samples were transported from the out-patient clinic to the influenza surveillance laboratory within 2 h for virus isolation using both MDCK cell culture method and specific pathogen-free (SPF) 9–11-day-old embryonated chicken egg culture method (Schwiger et al. 2000). Further subtyping of the strains, i.e., lineages of B/Yamagata (B/Y) and B/Victoria (B/V) was performed at the National Influenza Center, China by using hemagglutination inhibition assay, and then samples were stored at −80°C until RNA isolation.
Virus RNA extraction
All influenza B viruses used in this study were re-cultured in MDCK cells for two passages before RNA extraction using a commercial available viral RNA kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol.
Primer and probe design
Primers and probes for influenza virus B were selected using the MEGA software program (Version 3.1; http://www.megasoftware.net). The specific primers and probe sets for B/Y were designed using the Primer Premier (Version 5.0; PREMIER Biosoft International, CA, http://www.premierbiosoft.com) based on sequences comparison among over 50 Yamagata lineage sequences belonging to the Yamagata clade located on the phylogenetic tree of HA gene of influenza B virus constructed with MEGA software program (Version 3.1; http://www.megasoftware.net). The specific primer and probe sets for B/V were also designed as same as B/Y except for over 50 HA sequences belongings to the Victoria clade. The single nucleotide at the 3′ end of both primer pairs was specific for either B/Y or B/V. The other nucleotides of primer pairs and both probe sequences were complemented with both lineages. The B primer pair/probe set targeting on the NS gene of influenza B virus was modified from that described by Jie et al. (2009). The distinctive single nucleotide at the 3′ end of primer pairs and the designed primer and probe sequences were all compared with sequences submitted to the GenBank nucleotide database using BLASTN (Version 2.2.1; NCBI, MD, http://www.ncbi.nlm.nih.gov). The primers and probes employed in the present study were listed in Table 1.
One-step duplex real-time RT-PCR
One-step duplex real-time RT-PCR was performed with two specific primer sets designed to detect type B influenza virus and identify influenza B virus lineages: B/Y and B/V. A 25-μl PCR reaction contained 12.5 μl of 2× one-step RT-PCR buffer, 2.5 U TaKaRa Ex TaqTM HS, 0.5 μl PrimeScriptTM RT Enzyme Mix II (TaKaRa Biotechnology Co, Dalian, China), 5 μl RNA sample and two specific primer pair/probe sets, which comprised of primer pairs specific for B/V and B/Y lineages, respectively, at a final concentration of 0.5 μM, and their detection probes (HEX-labeled Bv, FAM-labeled By and CY5-labeled B) at concentrations of 0.2 μM for each. Amplification and detection were performed on ABI 7500 instrument (Applied Biosystems, Foster City, CA, USA) with the following conditions, 5 min at 42°C for reverse transcription, 10 s at 95°C for Taq HS activation, followed by 40 cycles for amplification with 95°C for 5 s and 60°C for 40 s. During amplification, the ABI detector monitored real-time PCR amplification by quantitatively analyzing fluorescence emissions. Results were collected and analyzed using the ABI 700 system SDS software (Version 1.3, Applied Biosystems).
Specificity of the duplex amplification
Influenza B virus isolated in our laboratory from 2006 to 2010 and confirmed by China National Influenza Center by HAI using specific serum raised against contemporary strains of either lineage were used as reference strains to determine the specificity of the duplex amplification. Influenza viruses A virus (including H1N1, H3N2, and novel H1N1 type) isolated and confirmed in the same period were used as negative control.
Sensitivity and reproducibility of the duplex amplification
The sensitivity of the duplex amplification was evaluated by viral load testing in terms of RNA copy number (Shisong et al. 2011). Briefly, the specific target gene segments of Yamagata lineage (B/Shenzhen/20/2006) and Victoria lineage (B/Shenzhen/102/2008) were amplified with PrimerScript one-step RT-PCR kit (TaKaRa Biotechnology Co.), respectively, using the primer pairs listed in Table 1. The amplified products were cloned into the pGEM-T Easy vector (Promega, Madison, WI) according to the manual and sequenced to verify its accuracy. The two plasmids containing the target segment of Yamagata lineage or Victoria Lineage were linearized by Sal I (New England Biolabs, Ipswitch, MA, USA) and gel-purified for use as templates with a RiboMax T7 Express In Vitro Transcription System (Promega, Madison, WI, USA) according to the manufacturer’s instruction. The concentrations of the in-vitro transcribed RNAs were determined with BioPhotometer (Eppendorf, Hamburg, Germany) and 10-fold serial dilutions of each transcribed RNA, ranging from 107 to 100 copies per microliter, were then prepared. The sensitivity and reproducibility of the separate duplex real-time RT-PCR were as those described previously (Wu et al. 2008). The sensitivity and reproducibility for the detection of Influenza B virus in multiplex real-time RT-PCR system were reported earlier by our laboratory (Shisong et al. 2011).
Results
Virological surveillance
In 2006–2010, total 17,765 throat swab samples of ILI cases were obtained from 10 hospitals. Two thousand, five hundred eighty-nine influenza virus strains were isolated by using either MDCK cell culture or SPF embryonated chicken egg culture. These virus stains included 425 H1N1, 750 H3N2, 152 Yamagata, 641 Victoria, and 621 novel H1N1, which were confirmed with HAI assay by China National Influenza Center. These results were in agreement with our results when these virus strains were firstly isolated by our laboratory. The annual distribution of influenza viruses with the rate of culture confirmed cases in 2006–2010 was shown in Fig. 1.
Annual distribution of influenza isolates based on both cell culture and embryonated chicken egg culture from 2006 to 2010, together with the positive rate of culture confirmed cases. The positive rate indicates the percentage of the number of influenza virus culture positive samples divided by the total throat swab specimens
One-step duplex real-time RT-PCR detection for influenza B virus, Victoria lineage and influenza B virus, Yamagata lineage
Two separate one-step duplex real-time RT-PCR systems were developed to differentiate simultaneously the B/Y and B/V lineages (Figs. 2 and 3). Influenza B virus was detected in both systems for which influenza B virus played a role of positive control in each differentiation system at the same time. In every duplex detection system, two sets of primers and probes specific for NA gene of influenza B virus and HA gene of Yamagata lineage (or Victoria lineage) were used. The primers and probes were designed to work simultaneously under the same PCR condition. The results as shown in Figs. 2 and 3 indicated that a dual fluorescent signal specific to influenza B virus and Victoria lineage or influenza B virus and Yamagata lineage, respectively, could be detected in each duplex amplification system.
Assay specificity and sensitivity
The specificity of the duplex RT-PCR was assessed by testing reference strains of subtypes of influenza type B and subtypes H1N1, H3N2, and H5N1 (Shisong et al. 2011), which to a large extent guaranteed the specificity in the duplex detection system only if a dual fluorescent signal for influenza B virus and Yamagata (or Victoria) substrain, respectively, appeared simultaneously, although other respiratory reference strains were not considered in this system, such as adenovirus, parainfluenza virus, respiratory syncytial virus, etc. The primers and probe used in this study for influenza B virus detection were as same as those had been used in another quadruplex real-time RT-PCR syste as described previously (Shisong et al. 2011). A total of 227 A-type virus strains (14 for H1N1, 42 for H3N2, and 171 for novel H1N1) obtained in 2010 were further tested. Our method to assess the possibility of false-positive results indicated that none of the samples gave a positive signal, true-negative/specificity reached to 100%.
The sensitivity of each duplex RT-PCR was determined by serial 10-fold dilutions of each virus sample, RNAs ranging from 107–100 copies per microliter, which was amplified using the duplex PCR assay. Less than 52.5 copies per microliter for B/V and 47 copies per microliter for B/Y could be detected by using this duplex real-time RT-PCR system (Figs. 4 and 5). This level of sensitivity correlated with 10−2 TCID50 of influenza virus A and 10−2 TCID50 of influenza Virus B, indicating that this method is of high sensitivity to identify influenza B virus lineages.
Efficiency and reproducibility
The issue of reproducibility was addressed by comparison of a number of separate experimental assays. First, the reproducibility of sample loading and detection procedure was examined by analyzing standard curve of HA gene for either B/V or B/Y obtained by plotting their cycle threshold numbers (C t) against their dilution factors (107–102 copies per microliter). Comparison of each duplex amplification demonstrated that the assay was able to generate high values of correlation (R 2 > 0.99) between C t values and dilution factors. By calculating the slope rate of the linear regression, the amplification efficiencies of HA gene for B/V and B/Y substrains based on RNA transcription in vitro by using in the duplex real-time RT-PCR assay were 110.368% and 117.69%, respectively, suggesting that the target gene was high-efficiently amplified by each duplex assay. The coefficient of variation obtained from each dilution, ranging from 107–102 copies per microliter, in independent sextuplicate reactions and 2-fold duplicate reactions. As shown in Table 2, the intra-CV and inter-CV for B/V were 0.64–1.20% and 1.72–3.39%, respectively, whereas the intra-CV and inter-CV for B/Y were 0.88–1.65% and 1.08–2.94%, respectively, indicating that the duplex assay was reliable with high reproducibility in amplification of HA gene for Victoria or Yamagata lineages.
Clinical applicability
To evaluate potential clinical applicability of this assay, total 793 confirmed positive B virus throat swabs samples obtained in 2006–2010 were retested with the duplex real-time RT-PCR. Results were comparable with HAI method, shown in Table 3.
Discussion
Viral culture in combination with HAI is still the “gold standard” for typing and subtyping of influenza virus (Ellis and Zambon 2002), the weakness of long time consumption (in general, it takes 3 to 7 days) limits its usefulness in quick public health responses to potential epidemics and even pandemics. Presently, more and more diagnostic methods are being developing for identifying influenza viruses including direct immunoassay, antibody testing (Naolo et al. 1999), one-step RT-PCR, multiplex RT-PCR (Sonia et al. 2007), electronic microarray (Ying et al. 2009), melting curve analysis (Toshimasa et al. 2008), real-time RT-PCR (Van et al. 2001), NASBA (Van et al. 2006), and LAMP (Masahiro et al. 2006). Among them, real-time RT-PCR has become the main viral detection technique in the event of a pandemic outbreak on account of its advantages of convenience, high sensitivity, low carryover contamination, and large-scale applicability. However, few lineage differentiation methods based on real-time RT-PCR for influenza V virus have been described, with the exception of using Taqman MGB probes incorporating lineage-specific nucleotides within probe sequences, which may generate some false signals to a certain extent once any nucleotide changes making it necessary to redesign and retest the probes.
In the case of influenza B virus, the current Yamagata and Victoria lineages display high intra- or inter-lineage sequence identity. Their variants usually appear with a point mutation of a nucleotide in the HA1 gene, leading to one amino acid substitution (Nakagawa et al. 2005). Evolutionary changes in antigenicity within and between distinct Yamagata and Victoria lineages of influenza B virus were reported as a result of changes in herd immunity with a model of positive selection. Usually, the variant sites are predominantly located on or near four major epitopes on HA1: the 120-loop, the 150-loop, the 160-loop, and the 190-helix (Chen and Edward 2008; Jun et al. 2009). There are few stable lineage-specific nucleotides in other regions of the HA1 sequence.
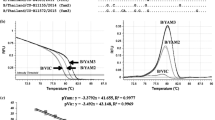
With any nucleic acid amplification method, accuracy can be affected by the presence of single nucleotide polymorphisms (SNP) beneath primers or probes, careful analysis, and utilization of degenerate and modified nucleotide bases can minimize the effects of sequence variants(Hamas et al. 2008; Hymas et al. 2007). It is recognized that molecular evolutionary characterization of the HA gene of influenza B virus provides a basis on which the lineages can be differentiated using specifically designed primers with one single nucleotide mismatch at the 3′ end rather than mismatched nucleotides occurring inside the probe sequences. In the early experiments, two sets of primers targeting on the HA gene of Yamagata and Victoria lineages, respectively, were evaluated regarding their specificity in RT-PCR amplification followed by gel electrophoresis and melting curve analysis with SYBR Green I.
The difference between the pair of primers designed for each lineage only lies in the nucleotide complementary type ((A vs. T) or (C vs. G)) selected as the lineage-specific SNP at the 3′ end of primer pairs or at least one primer. Specific signal was generated only when at least one of the primers carried a (A or T) at the 3′ end (shown in S1 and S2 as supplement). If both of the primers carried (C or G) at the 3′ end, no matter what lineage RNA was used as template, the dual signals were invariably generated in the above methods, which indicated that the Yamagata and Victoria lineages could not be differentiated with each other.
In summary, the present study has reported the first application of one-step duplex real-time RT-PCR for the differentiation of influenza B virus lineages on the basis of single nucleotide mismatch at the 3′ end of primers. The high specificity and good reproducibility of this assay enables its usage by most influenza surveillance network laboratories not only for viral diagnostics but also for on-time epidemiological examination for WHO decisions on vaccine composition, thereby speeding up the response to influenza outbreaks.
References
Biere B, Bauer B, Schweiger B (2010) Differentiation of influenza B lineages Yamagata and Victor by real-time PCR. J Clin Microbiol 48(4):1425–1427
Chen R, Edward CH (2008) The evolutionary dynamics of human influenza B virus. J Mol Evol 66(6):655–663
Ellis JS, Zambon MC (2002) Molecular diagnosis of influenza. Rev Med Viol 12(6):375–389
Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwann GF, Olsen B, Osterhaus AD (2005) Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol 79(5):2814–2822
Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, López-Gatell H, Olivera H, López I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ (2009) Antigenic and genetic characteristics of swine-origin 2009 (H1N1) Influenza viruses circulating in Humans. Scinece 325(197):197–201
Gavin JD, Smith DV, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A (2009) Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459(7250):1122–1126
Hamas WC, Aldous WK, Taggart EW, Stevenson JB, Hillyard DR (2008) Description and validation of a novel real-time RT-PCR enterovirus assay. Clin Chem 54(2):406–413
Holmes EC, Taubenberger JK, Grenfell BT (2005) Heading off an influenza pandemic. Science 309(5737):989
Hymas W, Atkinsong A, Stevenson J, Hillyard D (2007) Use of modified oligonucleotides to compensate for sequence polymorphoisms in the real-time detection of norovirus. J Virol Methods 142(1–2):10–14
Jie H, Bose ME, Beck ET, Fan J, Tiwari S, Metallo J, Jurgens LA, Kehl SC, Ledeboer N, Kumar S, Weisburg W, Henrickson KJ (2009) Rapid Multiplex RT-PCR typing of influenza A and B, and subtyping of influenza A into H1, 2, 3, 5, 7, 9, N1 (human), N1 (animal), N2 and N7 including typing of novel swine-origin Influenza A (H1N1) virus during current 2009 outbreak in Milwaukee, Wisconsin. J Clin Microbiol 47(9):2772–2778
Joanna SE, Joanne WS, Sharleen B, Matthew L, Katrina B, Maria CZ (2007) Design and validation of an H5 TaqMan real-time one-step reverse transcription-PCR and confirmatory assays for diagnosis and verification of influenza A virus H5 infections in humans. J Clin Microbiol 45(5):1535–1543
Jun S, Brian DK, Jianpeng M, Qinghua W (2009) Diversifying selection pressure on influenza B virus hemagglutinin. J Med Virol 81(1):114–124
Kanegae Y, Sutiga S, Endo A, Ishide M, Senya S, Osake K, Nerome K, Oya A (1990) Evolutionary pattern of the hemagglutinin gene of influenza B virus isolated in Janpa: cocirculating lineages in the same epidemic season. J Virol 64(6):2860–2865
Kingsford C, Nagarajan N, Salzberg SL (2009) 2009 swine-origin influenza A(H1N1) resembles previous influenza isolates. PLoS One 4(7):1–6
Masahiro I, Masahiro W, Naoko N, Toshiaki I, Yoshinobu O (2006) Rapid detection and typing of influenza A and B by loop-mediated isothermal amplification:comparison with immunochromatography and virus isolation. J Virol Methods 135(2):272–275
Muhammed Babakir M, Salvatore D, Carlo Federico P, Marco C (2009) Origin of the 2009 Mexico influenza virus: a comparative phylogenetic analysis of the principal external antigens and matrix protein. Arch Virol 154(8):1349–1352
Nakagawa N, Maeda A, Kase T, Kubota R, Okuno Y (1999) Rapid detection and identification of two lineages of influenza B strains with monoclonal antibodies. J Virol Methods 79(1):113–120
Nakagawa N, Kubota R, Okuno Y (2005) Variation of the conserved neutralizing epitope in influenza B virus Victoria group isolates in Japan. J Clin Microbiol 43(8):4212–4214
Nelson MI, Simonsen L, Viboud C, Miller MA, Taylor J, St. George K, Griesemer EG, Ghedin E, Sengamalay NA (2006) Stochastic processes are key determinants of short-term evolution in influenza A virus. PLoS Pathog 2(12):e125
Petric M, Comanor L, Petti CA (2006) Role of the laboratory in diagnosis of influenza during seasonal epidemics and potential pandemics. J Infect Dis 194:S98–S110
Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K (1990) Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 175(1):59–68
Schwiger B, Zadow I, Heckler R, Timm H, Pauli G (2000) Application of a fluorogenic PCR assay for typing and subtyping of influenza viruses in respiratory samples. J Clin Microbiol 38(4):1552–1558
Shisong F, Jianxiong L, Xiaowen C, Cunyou Z, Ting W, Xing L, Xin W, Chunli W, Renli Z, Jinquan C, Hong X, Muhua Y (2011) Simultaneous detection of influenza virus type B and influenza A virus subtypes H1N1, H3N2, and H5N1 using multiplex real-time RT-PCR. Appl Microbiol Biotechnol 90(4):1463–1470
Sonia EL, Josue IO, Lance FBT, James MB, Robert WD, Stuart C, Dannelle M, Mary TM (2007) Multiplexed reverse transcriptase PCR assay for identification of viral respiratory pathogens at the point of care. J Clin Microbiol 45(11):3498–3505
Stohr K (2002) Influenza-WHO cares. Lancet Infect Dis 2:517
Suwannakarn K, Payungporn S, Chieochansin T, Samransamruajkit R, Amonsin A, Songserm T, Chaisingh A, Chamnanpood P, Chutinimitkul S, Theamboonlers A, Poovorawan Y (2008) Typing (A/B) and subtyping (H1/H3/H5) of influenza A viruses by multiplex real-time RT-PCR assays. J Virol Methods 152(1–2):25–31
Toshimasa N, Nstsumi H, Naoko N (2008) Detection of antigenic variants of the influenza B virus by melting curve analysis with LCGreen. J Virol Methods 148(1–2):296–299
Van E, Nijhuis M, Schipper P, Schuurman R, Van AM (2001) Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol 39(1):196–200
Van AP, BrengeI-Pesce K, Lefeuvre A (2006) Real-time NASBA assay for the detection of influenza A and B. J Clin Virol 36(3):46–47
WHO (2011) http://www.who.int/csr/disease/influenza/recommendations_2011_12north/en/index.html
Wu C, Cheng X, He J, Lv X, Wang J, Deng R, Long Q, Wang X (2008) A multiplex real-time RT-PCR for detection and identification of influenza virus types A and B and subtypes H5 and N1. J Virol Methods 148(1–2):81–88
Ying H, Huong T, Stuart D, Yuwen H, Sylvia N, Msdhu G, Jie H, Michael B, Kelly JH, Jinag F, Andrea JK (2009) Multiplex assay for simultaneously typing and subtyping Influenza viruses by use of an electronic microarray. J Clin Microbiol 47(2):390–396
Acknowledgements
We thank China National Influenza Centre for their technical support and confirmation to these two real-time RT-PCR systems.
Author information
Authors and Affiliations
Corresponding authors
Additional information
This study was supported by Science and Technology grant 2008B080702016 from Guangdong Provincial Department of Science and Technology.
Naixing Zhang and Shisong Fang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 48 kb)
Rights and permissions
About this article
Cite this article
Zhang, N., Fang, S., Wang, T. et al. Applicability of a sensitive duplex real-time PCR assay for identifying B/Yamagata and B/Victoria lineages of influenza virus from clinical specimens. Appl Microbiol Biotechnol 93, 797–805 (2012). https://doi.org/10.1007/s00253-011-3710-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3710-8