Abstract
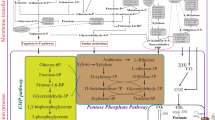
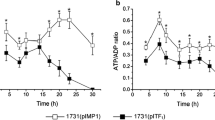
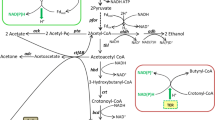
Fermentative production of solvents (acetone, butanol, and ethanol) by Clostridium acetobutylicum is generally a biphasic process consisting of acidogenesis and solventogenesis. We report that the biphasic metabolism of C. acetobutylicum could be changed by oxidoreduction potential (ORP) regulation. When using air to control the ORP of the fermentation broth at −290 mV, an earlier initiation of solventogenesis was achieved. Solvent production reached 25.6 g l−1 (2.8 g acetone l−1, 16.8 g butanol l−1, 6.0 g ethanol l−1), a 35% increase compared with the ORP uncontrolled process. Metabolic flux analysis revealed that there was a general increase of the central carbon flux in the first 24 h of fermentation when ORP was controlled at −290 mV, compared with the control. Specifically, the solvent ratio (acetone:butanol:ethanol) was changed from 25:64:11 to 11:66:23 at ORP level of −290 mV, which might have resulted from the rigidity at acetyl-CoA node and the flexibility at acetoacetyl-CoA and butyryl-CoA nodes in response to ORP regulation.




Similar content being viewed by others
References
Assobhei O, Kanouni AE, Ismaili M, Loutfi M, Petitdemange H (1998) Effect of acetic and butyric acids on the stability of solvent and spore formation by Clostridium acetobutylicum ATCC 824 during repeated subculturing. J Ferment Bioeng 85(2):209–212
Bahl H, Gottwald M, Kuhn A, Rale V, Andersch W, Gottschalk G (1986) Nutritional factors affecting the ratio of solvents produced by Clostridium acetobutylicum. Appl Environ Microbiol 52(1):169–172
Bourel G, Henini S, Divies C, Garmyn D (2003) The response of Leuconostoc mesenteroides to low external oxidoreduction potential generated by hydrogen gas. J Appl Microbiol 94(2):280–288
Buday Z, Linden JC, Karima MN (1990) Improved acetone–butanol fermentation analysis using subambient HPLC column temperature. Enzyme Microb Technol 12(1):24–27
Desai RP, Nielsen LK, Papoutsakis ET (1999) Stoichiometric modeling of Clostridium acetobutylicum fermentations with non-linear constraints. J Biotechnol 71(1–3):191–205
Girbal L, Soucaille P (1994) Regulation of Clostridium acetobutylicum metabolism as revealed by mixed-substrate steady-state continuous cultures: role of NADH/NAD ratio and ATP pool. J Bacteriol 176(21):6433–6438
Girbal L, Vasconcelos I, Saint-Amans S, Soucaille P (1995) How neutral red modified carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture. FEMS Microbiol Rev 16(2–3):151–162
Harris LM, Welker NE, Papoutsakis ET (2002) Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J Bacteriol 184(13):3586–3597
Hartmanis MG, Gatenbeck S (1984) Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 47(6):1277–1283
Hirsch A, Grinsted E (1954) Methods for the growth and enumeration of anaerobic spore-formers from cheese, with observations on the effect of nisin. J Dairy Res 21:101–110
Husson F, Tu VP, Santiago-Gomez M, Cachon R, Feron G, Nicaud JM, Kermasha S, Belin JM (2006) Effect of redox potential on the growth of Yarrowia lipolytica and the biosynthesis and activity of heterologous hydroperoxide lyase. J Mol Catal B Enzym 39:179–183
Ishizaki A, Snibai H, Hirose Y (1974) Basic aspects of electrode potential change in submerged fermentation. Agric Biol Chem 38:2399–2405
Jones DT, Woods DR (1986) Acetone–butanol fermentation revisited. Microbiol Rev 50(4):484–524
Junelles AM, Janati-Idrissi R, Petitdemange H, Gay R (1988) Iron effect on acetone–butanol fermentation. Curr Microbiol 17:299–303
Kim O, Rakesh B, Eugene LI (1988) Redox potential in acetone–butanol fermentations. Appl Biochem Biotechnol 18(1):175–186
Lutke-Eversloh T, Bahl H (2011) Metabolic engineering of Clostridium acetobutylicum: recent advances to improve butanol production. Curr Opin Biotechnol 22:1–14
Meyer CL, Papoutsakis ET (1989) Increased levels of ATP and NADH are associated with increased solvent production in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 30(5):450–459
Meyer CL, Roos JW, Papoutsakis ET (1986) Carbon monoxide gasing leads to alcohol production and butyrate uptake without acetone formation in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 24:159–167
Ouvry A, Wache Y, Tourdot-Marechal R, Divies C, Cachon R (2002) Effects of oxidoreduction potential combined with acetic acid, NaCl and temperature on the growth, acidification, and membrane properties of Lactobacillus plantarum. FEMS Microbiol Lett 214(2):257–261
Papoutsakis ET (1984) Equations and calculations for fermentations of butyric acid bacteria. Biotechnol Bioeng 26(2):174–187
Peguin S, Goma G, Delorme P, Soucaille P (1994) Metabolic flexibility of CIostridium acetobutylicum in response to methyl viologen addition. Appl Microbiol Biotechnol 42:611–616
Rao G, Mutharasan R (1986) Alcohol production by Clostridium acetobutylicum induced by methyl viologen. Biotechnol Lett 8(12):893–896
Rao G, Mutharasan R (1987) Altered electron flow in continuous cultures of Clostridium acetobutylicum induced by viologen dyes. Appl Environ Microbiol 53(6):1232–1235
Rao G, Mutharasan R (1989) NADH levels and solventogenesis in Clostridium acetobutylicum: new insights through culture fluorescence. Appl Microbiol Biotechnol 30(1):59–66
Rathin D, Zeikus JG (1985) Modulation of acetone–butanol–ethanol fermentation by carbon monoxide and organic acids. Appl Environ Microbiol 49(3):522–529
Ravagnani A, Jennert KC, Steiner E, Grunberg R, Jefferies JR, Wilkinson SR, Young DI, Tidswell EC, Brown DP, Youngman P, Morris JG, Young M (2000) Spo0A directly controls the switch from acid to solvent production in solvent-forming clostridia. Mol Microbiol 37(5):1172–1185
Riondet C, Cachon R, Wache Y, Alcaraz G, Divies C (1999) Changes in the proton-motive force in Escherichia coli in response to external oxidoreduction potential. Eur J Biochem 262:595–599
Riondet C, Cachon R, Wache Y, Alcaraz G, Divie C (2000) Extracellular oxidoreduction potential modifies carbon and electron flow in Escherichia coli. J Bacteriol 182(3):620–626
Senger RS, Papoutsakis ET (2008) Genome-scale model for Clostridium acetobutylicum: part I. Metabolic network resolution and analysis. Biotechnol Bioeng 101(5):1036–1052
Sillers R, Chow A, Tracy B, Papoutsakis ET (2008) Metabolic engineering of the non-sporulating, non-solventogenic Clostridium acetobutylicum strain M5 to produce butanol without acetone demonstrate the robustness of the acid-formation pathways and the importance of the electron balance. Metab Eng 10(6):321–332
Sillers R, Al-Hinai MA, Papoutsakis ET (2009) Aldehyde-alcohol dehydrogenase and/or thiolase overexpression coupled with CoA transferase downregulation lead to higher alcohol titers and selectivity in Clostridium acetobutylicum fermentations. Biotechnol Bioeng 102(1):38–49
Sridhar J, Eiteman MA (1999) Influence of redox potential on product distribution in Clostridium thermosuccinogenes. Appl Biochem Biotechnol 82:91–101
Tomas CA, Welker NE, Papoutsakis ET (2003) Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell's transcriptional program. Appl Environ Microbiol 69(8):4951–4965
Wang S, Zhang Y, Dong H, Mao S, Zhu Y, Wang R, Luan G, Li Y (2011) Formic acid triggers the “acid crash” of acetone–butanol–ethanol fermentation by Clostridium acetobutylicum. Appl Environ Microbiol 77(5):1674–1680
Yerushalmi L, Volesky B, Szczesny T (1985) Effect of increased hydrogen partial pressure on the acetone–butanol fermentation by Clostridium acetobutylicum. Appl Microbiol Biotechnol 22:103–107
Zhang Y, Wang S, Liu W, Li Y (2010) Equipment for automatic control of oxidoreduction potential and its utilization. China Patent 201010593474.3
Acknowledgements
This work was supported by the National High Technology Research and Development Program of China (No. 2011AA02A208), the National Natural Science Foundation of China (Grant No. 30900012), and the Scientific Equipment R&D Program of the Chinese Academy of Sciences (No. YZ200941). Yin Li is supported by the Hundreds Talents Program of the Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, S., Zhu, Y., Zhang, Y. et al. Controlling the oxidoreduction potential of the culture of Clostridium acetobutylicum leads to an earlier initiation of solventogenesis, thus increasing solvent productivity. Appl Microbiol Biotechnol 93, 1021–1030 (2012). https://doi.org/10.1007/s00253-011-3570-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3570-2