Abstract
Currently available enterotoxigenic Escherichia coli (ETEC) vaccines are based on colonization factors and/or the heat-labile enterotoxin B subunit (LTB). However, the induction of antitoxic responses against heat-stable enterotoxin a (STa) and b (STb) has merit as these two poorly immunogenic toxins are frequently associated with ETEC strains. In this study, we genetically constructed a trivalent enterotoxin fusion protein (STa–LTB–STb, abbreviated to SLS) in an effort to develop a single toxoid containing these three enterotoxins for vaccination against ETEC. Mutagenesis at one disulfide-bridge-forming cysteine in STa led to a dramatic reduction in the STa toxicity of SLS; however, the fusion peptide retained the STb-associated toxicity. Immunization of mice with SLS protein elicited significant antibody responses to LTB, STa, and STb. Significantly, the mice antisera were able to neutralize the biological activity of both STa and STb. In the experiment to assess the protective effect of SLS immunization, the mortality of mice receiving SLS was significantly lower than their control cohorts (P < 0.01) after intraperitoneal challenge with ETEC. These results show that the trivalent fusion enterotoxin SLS has the potential to serve as a useful toxin-based vaccine against ETEC-induced diarrheal disease via a single immunogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enterotoxigenic Escherichia coli (ETEC) is the most common cause of severe diarrhea in young animals and results in considerable morbidity and mortality in piglets and calves (Nagy and Fekete 2005). The virulence factors of ETEC include adhesins and enterotoxins. Adhesins including fimbriae and afimbrial proteins mediate the binding of the bacteria to enterocytes, an event essential for colonization, proliferation, and progression of ETEC infections (Erume et al. 2008). ETEC strains mainly express two types of enterotoxins: heat-labile enterotoxin (LT) subdivided into LT-I and LT-II, and heat-stable enterotoxins (ST) subdivided into STa and STb according to differences in protein structure and pathogenesis (Dubreuil 1997). Since LT-II is infrequently detected and nearly always nonpathogenic (Nataro and Kaper 1998), most vaccine development work has focused on LT-I. LT-I is similar in structure and function (approximately 80% identity in primary sequence) to the cholera toxin (CT) expressed by Vibrio cholerae as both are ADP-ribosylating enterotoxins and consist of a subunit A (LTA, 27 kDa) and five identical B subunits (LTB, 11.6 kDa) forming a receptor-binding pentamer (Nataro and Kaper 1998; Rosales-Mendoza et al. 2009). STs are low molecular- weight polypeptides, where STa (2 kDa) is an 18- (porcine or human origin) or 19- (human origin) amino-acid peptide and functions by stimulating guanylate cyclase C (Nataro and Kaper 1998), and STb (5.2 kDa) (Kupersztoch et al. 1990) is a 48-amino-acid peptide and functions by acting on sulfatide, an acidic glycosphingolipid on intestinal epithelial cells (Rousset et al. 1998).
Although adhesin-mediated colonization is important in ETEC pathogenesis, enterotoxins are thought to be the central virulence factors causing diseases and may also play a role in the colonization process (Allen et al. 2006; Berberov et al. 2004; Zhang et al. 2006). Overall, LT-, STa-, and STb-producing strains are very common, having been shown to be present in approximately 50%, 75% (Sizemore et al. 2004), and 90% (Dubreuil et al. 1996) of ETEC isolates, respectively. An ideal vaccine should provide protective immunity against both adhesin antigens and enterotoxins. In addition to the more than 25 recognized colonization factors from human-associated strains (Gaastra and Svennerholm 1996; Svennerholm and Tobias 2008) and over eight main types of fimbriae from animal-associated strains (Nagy and Fekete 2005), there are large numbers of ETEC isolates that do not express any of the known colonization factors (Dubreuil et al. 1996; Qadri et al. 2005), a factor that may limit the efficacy of colonization factor-based ETEC vaccines. On the other hand, considering the limited number of the classes of enterotoxins and their decisive role in pathogenesis, the induction of antitoxic immunity may be an effective way to improve the protective effect of ETEC vaccines (Sizemore et al. 2004; Steinsland et al. 2003). Therefore, many current ETEC vaccines include LTB or CTB (subunit B of CT) in an effort to augment immune responses. STs toxoids would be desirable for preventive vaccination but their developments have faced serious hurdles due to their lack of immunogenicity (Wolf 1997). Thus far, no effective nontoxic STa or STb toxoid capable of eliciting neutralizing antibodies has been constructed or included in commercial vaccines for humans (Sizemore et al. 2004; Svennerholm and Tobias 2008). STs are poorly immunogenic due to their small size; however, they can attain immunogenicity when coupled chemically or genetically to an appropriate carrier. In order to develop desirable STa or STb toxoid and induce specific anti-STs immunity, numerous studies have been conducted to link STs to a large carrier molecule, such as LTB, CTB, OmpC (E. coli outer membrane protein C), IgG-binding fragment, maltose binding protein and OmpF, and β-galactosidase (Cardenas and Clements 1993; Clements 1990; Dubreuil et al. 1996; Lawrence et al. 1990; Lowenadler et al. 1991; Rosales-Mendoza et al. 2009; Saarilahti et al. 1989; Sanchez et al. 1988; Zhang et al. 2010). These studies usually constructed a bivalent fusion in an ST-carrier mode with varied immunogenicity and residual toxicity.
The goal of this study was to construct a trivalent enterotoxin fusion gene of STa–LTB–STb and produce the corresponding polypeptides carrying antigenic determinants of STa, STb, and LTB for use as an immunogen to provide broad-spectrum protection against ETEC.
Materials and methods
Bacterial strains, media, and plasmids
ETEC F4ac strain C83902 (O8:K87; LT+, STa+, STb+) and F5 strain C83914 (O101:K30; LT−, STa+, STb-) were obtained from China Institute of Veterinary Drug Control (Beijing, China). F4ac strains were cultured in CAYE medium (Mundell et al. 1976) prior to use in the challenge study with mice. F5 strains were grown in the asparagine–salt medium (Staples et al. 1980) for neutralization assay. The vector pET-30a (+) and pET-32a (+) were products of Novagen (Gibbstown, NJ, USA) and used for the expression of recombinant enterotoxins.
Construction of recombinant enterotoxin expression vectors
The gene for LTB or STb was amplified from E. coli F4ac strain heat lysate, with the STa gene being synthesized by splicing by overlap extension (SOE) polymerase chain reaction (PCR) without using a template. To reduce the toxicity, the cysteine (tgc) at position 14 of STa was mutated to alanine (gcc) through an alteration of a codon (gca→ggc) on primer STa-R. Then, the enterotoxin genes LTB, STa, and STb were fused genetically by SOE PCR to produce the polyvalent fusion gene: STa–LTB–STb (denoted SLS). The PCR products of SLS were purified and cloned into U-vector pDrive (Qiagen, Mississauga, Ontario, Canada), and the resulting plasmid, pDrive-SLS, was used as the source of the gene for SLS, LTB, or STb for the remainder of this study. Subsequently, the recombinant enterotoxin expression plasmids pET30-SLS and pET30-LTB were constructed by inserting the double-digested PCR-amplified fragments SLS and LTB into plasmid pET-30a digested with the corresponding two restriction enzymes. Both plasmids encoded their respective recombinant enterotoxin plus a His6 tag at the C-terminus. Similarly, plasmid pET32-STb was constructed by inserting a mature STb gene into the pET-32a vector within the EcoRI and XhoI sites. This plasmid encoded a thioredoxin (Trx) tag, His6 tag, and S tag at the N-terminus of the inserted STb sequence and another His6 tag at the C-terminus. All the PCR primers used are shown in Table 1.
Recombinant enterotoxins production
Recombinant E. coli BL21 (DE3) strains harboring plasmids pET30-SLS, pET30-LTB, or pET32-STb were induced to express fusion enterotoxins by the addition of 1 mM isopropyl β-d-thiogalactoside (IPTG). The E. coli cells were collected and disrupted by sonication and then centrifuged to isolate the inclusion bodies. The inclusion bodies of SLS, LTB, or Trx-STb were washed thoroughly with Tris-EDTA buffer containing Triton X-100 or urea (Sambrook and Russell 2001) and stored at −20 °C until use. The purity of the washed inclusion bodies of fusion protein was determined by SDS-PAGE analysis using NIH software ImageJ (Version 1.41). For immunization, washed insoluble fusion enterotoxins were solubilized with 1% SDS and then dialyzed against phosphate-buffered saline (PBS) to remove the detergent. For precoating the microtiter plates in enzyme-linked immunosorbent assay (ELISA), washed insoluble fusion enterotoxins were solubilized with 8 M urea.
SDS-PAGE and Western blot analysis
Samples of the total cell protein, the soluble cytoplasmic fraction, and the insoluble cytoplasmic fraction of the induced E.coli BL21 strains were respectively separated on a 15% polyacrylamide gel according to the method of Laemmli (1970). Gels were either stained with Coomassie brilliant blue R-250 or electroblotted to polyvinylidene fluoride membranes (0.45 μm pore size, Millipore, Bedford, MA, USA). Proteins on the membranes were probed first with primary antibodies, 1:1,000 diluted anti-His antibody (TIANGEN BIOTECH, Beijing, China) and then with horseradish peroxidase-conjugated goat anti-mouse IgG (H+L) (Beyotime, Jiangsu, China) diluted at 1:2,000. Positive protein bands were detected upon development with BeyoECL Plus kit (Beyotime).
Mice immunization and challenge
Forty female, 8–10-week-old Kunming mice (Animal Center, Dalian Medical University, Dalian, China) were randomly allocated into four groups with ten mice/group and housed in ventilated cages. The mice were immunized intraperitoneally with 100 μg of solubilized fusion protein SLS, 60 μg of solubilized fusion protein LTB, 3×108 killed F4ac whole cells inactivated by multiple freeze–thaw cycles, or PBS as a negative control. Each of the antigens was adjusted to a final volume of 0.3 ml with sterile PBS. The killed ETEC F4ac preparation was checked for sterility by streaking on LB agar. The mice were immunized three times at 2-week intervals and challenged intraperitoneally with 2×108 CFU of ETEC F4ac 2 weeks after the last immunization. The mice were monitored for morbidity for 14 days after the challenge. Blood was collected from the tail vein 2 days before each immunization and challenge.
Antibody ELISA
Specific antibodies against the three antigens STa, LTB, or STb were detected in the immunized mice sera by ELISA. Briefly, 96-well microtiter plates (JET, Ontario, Canada) were coated with 1 μg/well of antigen protein, STa polypeptide (synthesized at Shanghai Bootech BioScience & Technology, Shanghai, China) in PBS (pH 7.2), fusion protein LTB, or STb in coating buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.6). After the blocking and washing steps, serial twofold dilutions (starting at 1:100) of sera were added to the wells and incubated for 1 h at 37 °C. Then, bound antibodies were detected by 60-min incubation with horseradish peroxidase- conjugated goat anti-mouse IgG (H+L) diluted 1:25,000 in PBS followed by tetramethyl benzidine substrate. Titers were determined as the log10 value of the highest serum dilution giving an absorbance of 0.2.
Enterotoxicity assay and neutralization
The residual enterotoxicity of fusion protein SLS was tested using the suckling mouse assay as described by Giannella (1976). Groups of three newborn suckling mice aged 2–3 days were inoculated intragastrically with 0.1-ml SLS polypeptide solution containing (testing the toxicity of STb) soybean trypsin inhibitor (2 mg/ml; Solarbio, Beijing, China) (Whipp 1990) or not (testing the toxicity of STa) and killed after 3-h inoculation. Pure STa polypeptide was used as a positive control for STa toxicity. One mouse unit (MU) was defined as the minimum effective dose of STs necessary to cause a positive response. G/C (weight ratio of gut to the remaining carcass) ratios of ≥ 0.090 were considered positive for toxicity of STa or STb.
To test the neutralization activity of the anti-SLS sera on the biological activity of STa and STb, two separate suckling mouse assay experiments were conducted: (1) neutralization of STa: ETEC F5 strains were grown in the asparagine–salt medium at 37 °C for 24 h with vigorous agitation, and the STa-containing supernatant was harvested and filtered through a 0.22-μm membrane filter (JET). Toxin–antibody mixtures were prepared by mixing 50 μl of fivefold diluted supernatant (containing about 5 MU STa) with 50 μl of serial twofold dilutions of pools of sera from mice immunized with SLS or E. coli F4ac. Following preincubation for 1 h at 37 °C, the mixture was administered to suckling mice; and (2) neutralization of STb: Fusion enterotoxin Trx-STb was purified by nickel affinity chromatography from the soluble cytoplasmic fraction of recombinant E. coli BL21 strain. Toxin–antibody mixtures were made by mixing 50 μl of solution containing 25 μg of purified Trx-STb protein solution (corresponding to approximately 5 MU Trx-STb) and 0.2 mg of soybean trypsin inhibitor with an equal volume of serial twofold dilutions of pools of sera from mice immunized with SLS or E. coli F4ac (Whipp 1990; Fujii et al. 1991). After preincubation for 1 h at 37 °C, the mixtures were injected to the suckling mice.
Statistical analysis
Antibody titers were compared by using nonparametric Kruskal–Wallis test followed by Dunn's post-test. Differences in survival data of mice were analyzed by the Kaplan–Meier log rank test with Holm–Sidak test for multiple comparisons.
Results
Construction of plasmids pET30-SLS, pET30-LTB, and pET32-STb
Plasmids pET30-SLS, pET30-LTB, and pET32-STb were constructed as shown in Fig.1. In order to decrease the toxicity of STa, the disulfide bond between cysteine6 and cysteine14, which is highly correlated to the toxicity of STa, was destroyed by substituting alanine for cysteine14. For the purpose of reducing the interaction among the fused three enterotoxins and retaining each component’s epitopes, two proline-rich oligopeptides, Pro-Pro-Ala-Ser-Pro and Ser-Ala-Ser-Thr-Thr-Pro-Pro, were included in the fusions between STa and LTB and LTB and STb, respectively. The plasmids were sequenced to confirm the correct insertion of the enterotoxin genes.
Construction of the E. coli recombinant enterotoxin expression vector pET30-SLS, pET30-LTB, and pET32-STb. Of the three enterotoxin genes, STa was synthesized by SOE (splicing by overlap extension) PCR, and initial LTB and STb were amplified from the E. coli F4ac strain. The univalent enterotoxin genes STa, LTB, and STb were fused to produce fusion gene STa–LTB–STb (SLS) via SOE PCR and the SLS was then cloned into U-vector pDrive to make pDrive-SLS. The genes for SLS, LTB, and STb were PCR-amplified from plasmid pDrive-SLS, digested with two enzymes, and then inserted into prokaryotic expression vector pET-30a or pET-32a digested with the appropriate enzymes to produce the final construct pET30-SLS, pET30-LTB, and pET32-STb
Expression and purification of the fusion enterotoxins
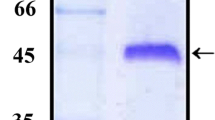
SDS-PAGE showed that, after induction with IPTG, the fusion proteins of approximately 21 kDa (SLS),13 kDa (LTB), and 24 kDa (Trx-STb) were produced as predicted by E. coli BL21 harboring plasmids pET30-SLS, pET30-LTB, and pET32-STb, respectively (Fig. 2a). Efforts to obtain soluble LTB and SLS proteins rather than inclusion bodies via culturing E. coli BL21 at a lower temperature, altering the concentration of IPTG as well as changing induction time proved unsuccessful. In contrast to LTB and SLS, Trx-STb was expressed in the form of both inclusion body and soluble cytoplasmic protein. The inclusion bodies of LTB, SLS, and Trx-STb were purified to > 85% purity by three washing steps as determined by SDS-PAGE analysis. Western blot showed that the fusion proteins, LTB, SLS, and Trx-STb, could react with anti-His antibodies (Fig. 2b).
Analysis of the expressed fusion enterotoxins using SDS-PAGE (a) and Western blot (b). a The samples of the total cell protein (T), soluble cytoplasmic fraction (S), and insoluble cytoplasmic fraction (I) of the induced recombinant E. coli BL21strains expressing SLS, LTB, and Trx-STb were loaded on a 15% gel from left to right. Lanes C1 and C2 (negative control) are the total cell protein of the induced E. coli BL21 and BL21-Trx (containing plasmid pET-32a), respectively. b Inclusion bodies of the fusion protein LTB (13 kDa), SLS (21 kDa), and Trx-STb (24 kDa) were detected by immunoblot using anti-His antibodies
Antitoxic antibody responses are elicited in mice
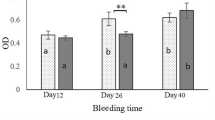
The serum antibody levels of the four groups of mice (n = 10) were evaluated after three immunizations using indirect ELISA (Fig. 3). In the SLS-vaccinated group, high levels of serum IgG were raised against LTB and hapten STa and STb, demonstrating that LTB is an effective carrier for STa and STb and bestowed good immunogenicity upon them. Based upon the values of log10 IgG titers, the antibody response to LTB [4.92 ± 0.28 (mean ± standard deviation)] was much higher than that to STa (3.52 ± 0.78) or STb (4.20 ± 0.67). In the LTB-vaccinated group, high levels of serum IgG were only produced against LTB (5.16 ± 0.32) with no IgG response to STa (1.00 ± 0.40) or STb (1.35 ± 0.32) being observed. In the F4ac-vaccinated group, the mice developed antibodies against LTB (3.81 ± 0.32) only, with no antibodies towards STa (1.22 ± 0.30) or STb (1.64 ± 0.31) being detected, demonstrating the poor immunogenicity of native STa and STb. No serum antibody responses against LTB, STa, or STb were detected in mice injected with PBS.
Mice serum IgG response 12 days after the third immunization as measured by ELISA against heat-labile enterotoxin subunit B (LTB), heat-stable enterotoxin a (STa), or heat-stable enterotoxin b (STb) antigens. Female Kunming mice (ten mice/group) were immunized with recombinant enterotoxin SLS (100 μg), LTB (60 μg), killed E. coli F4ac cells (3×108 CFU), or PBS (negative control) for three times at 2-week intervals. The antibody titer was determined as the highest serum dilution to reach an OD of 0.2. The mean ± SD for each group’s serum antibody titer is shown. *P < 0.001 compared with the control in each set of data against the respective antigen
LTB-specific antibody titers were only slightly lower in SLS-vaccinated mice as compared with LTB-vaccinated mice, with both groups exhibiting significantly higher (P < 0.001) titers than the PBS group. As for STa and STb, the SLS-vaccinated mice had high levels of antibody titers against both STa and STb which was significantly higher than any of the other groups (P < 0.001) except versus F4ac group at STb-specific antibody titers (P < 0.05). Therefore, the polyvalent enterotoxin SLS constructed in this study was effective in eliciting antitoxic antibody responses in mice to all of the constituent elements LTB, STa, and STb.
Neutralization of STa and STb toxicity
An important qualification for SLS protein being used as an immunoprophylactic was its capacity to elicit neutralizing antibodies against STs. The neutralizing potential of mice antisera to SLS was tested in the suckling mouse assay. Antisera to SLS fusion peptides (SLS–sera) exhibited a neutralizing activity against both STa and STb, with the control sera from mice receiving killed ETEC F4ac organisms having no such enterotoxin-neutralizing activity (Fig. 4). For neutralizing the selected dose (about 5 MU) of STa and STb, the highest dilutions of SLS–sera were 1/20 and 1/40, respectively.
Neutralization of STa (a) and STb (b) activity in the suckling mouse assay by antisera from mice immunized with fusion enterotoxin SLS (SLS–sera) or ETEC F4ac (Con–sera). The toxin–sera mixtures were made by mixing serially twofold diluted sera in PBS with an equal volume of STa-containing E. coli F5 culture supernatant (a) or trypsin inhibitor-containing thioredoxin–STb fusion protein solution (b) and incubating for 1 h at 37 °C. The mixtures were then inoculated orally into groups of three suckling mice. The mice were killed 3 h later, and the gut/carcass (G/C) weight ratios were measured. G/C ratios of ≥ 0.090 (indicated by a dotted line) are considered as positive for ST enterotoxicity
Toxicity of the fusion enterotoxin
In the absence of trypsin inhibitor, the highest dose tested of 20 μg of purified SLS protein did not induce a significant fluid accumulation within the intestinal tract of mice (G/C = 0.079). Since STa comprises approximately 10% (2/21 kDa) of the total weight of SLS, the actual amount of STa in the fusion protein was approximately 2 μg. This amount of STa is over 200 times greater than the minimum effective dose of pure STa (9 ng). This finding demonstrates that the mutated STa was virtually rendered non-toxic as a result of the amino acid substitution outlined above. However, the addition of trypsin inhibitor to 20 μg of SLS induced an obvious fluid secretion in the intestinal lumen (G/C = 0.133), indicating that the SLS protein still had the STb toxicity.
Immunization with polyvalent enterotoxin SLS provides protective immunity to mice against fatal ETEC challenge
To test whether immunization with polyvalent enterotoxin SLS could provide adequate protective immunity in vivo, the Kunming mice were used in a challenge study. Four groups (SLS, LTB, F4ac, and PBS) of ten female mice received a fatal dose of ETEC F4ac bacteria intraperitoneally 2 weeks after the third immunization. The incidences of clinical disease and mortalities were then recorded for 14 days (Fig. 5). Seven of ten SLS-vaccinated mice survived whereas all of the mice receiving PBS succumbed to the challenge (P < 0.01). Immunization with LTB provided an intermediate protection with four of ten mice surviving, a level that was still higher (P < 0.05) than the PBS group. In the F4ac-vaccinated group, eight mice died within 3 days post-challenge, and only two mice survived beyond 2 weeks.
Survival of the four groups of mice after triple immunization and challenge with E. coli F4ac strains. Kunming female mice (ten per group × four groups) were immunized three times with recombinant enterotoxin STa–LTB–STb (SLS), heat-labile enterotoxin subunit B (LTB), E. coli F4ac cells, or PBS. Two weeks after the third immunization, the mice were all challenged intraperitoneally with 2×108 ETEC F4ac organisms. The number of mice surviving on each day within 2 weeks of post-challenge is shown
Discussion
ETEC-mediated colibacillosis remains a major cause of mortality in young livestock all over the world. LT has already been included in many commercially available ETEC vaccines and STa has been the subject of numerous studies aiming to make a suitable STa toxoid (Cardenas and Clements 1993; Clements 1990; Rosales-Mendoza et al. 2009; Zhang et al. 2010). In contrast, STb has not been extensively studied for its potential as a vaccine component; this is likely due to the minor role that it plays in ETEC pathogenesis. STb is primarily associated with porcine ETEC strains and has only been sporadically detected in isolates of human, bovine, or chicken origin (Dubreuil 1997). For many years, the role of STb in causing diarrhea in animals, especially in piglets, has been controversial. In fact, although the presence of STb-positive ETEC and diarrhea in piglets is frequently observed, it often fails to reproduce a similar pathology in experimentally challenged animal models (Dubreuil 2008). The lack of use of STb in the current immunization program which contains just LT, colonization factors (e.g., F4, F5, F18, etc.), and potential STa may result in the blooming of STb-dependent colibacillosis (Dubreuil 2008). However, recent studies have defined the importance of STb (Berberov et al. 2004; Chapman et al. 2006) and clearly demonstrated that STb contributes to the development of diarrhea in piglets, although not as consistently as LT (Zhang et al. 2006). Given the recent findings implicating STb in ETEC pathogenesis, we elected to include it within our polyvalent enterotoxin.
To make the fusion enterotoxin SLS, we used recombinant DNA techniques rather than traditional chemical coupling approaches. The genetic fusion technique has been employed extensively in various fields to create multi-functional hybrid proteins comprising modules from various proteins (Crasto and Feng 2000; George and Heringa 2002). These modules are typically linked via oligopeptide linkers, the appropriate design of which is usually critical to the desired function of the hybrid protein. Several studies have analyzed the features and regular patterns of natural domain linkers and suggested some criteria for the selection of linkers (Argos 1990; Crasto and Feng 2000; George and Heringa 2002). A desirable linker usually adopts an extended conformation which can effectively separate the flanking domains and avoid interactions between them (Argos 1990; Tanaka et al. 2003). Moreover, the desirable linkers should be energetically and conformationally stable with little dependence on the rest of the domains; thus, the linker itself would not disturb the function of the joined domains (Argos 1990).
In this study, two linkers were required to link the three enterotoxins, and the design of these two linkers was of critical importance to ensure that the immunogenicity of all the three enterotoxins was retained. For our purposes, a short linker, Pro-Pro-Ala-Ser-Pro, originally designated as a “PRO type” by Argos (1990), was selected to join STa with LTB. A long linker, Ser-Ala-Ser-Thr-Thr-Pro-Pro, was selected to connect LTB and STb using the LINKER program established by Xue et al. (2004). These two linker peptides were selected for their extension and lack of major kinks or bends in conformation which was confirmed through visualizing them in their natural host polypeptides using Swiss-PdbViewer (Guex and Peitsch 1997). Proline is the most preferred amino acid in linker peptides and has been successfully used in linker peptides to join proteins in many studies (Cardenas and Clements 1993; Clements 1990; Dubreuil et al. 1996; Jagusztyn-Krynicka et al. 1993; Khan et al. 1994). The unique cyclic imino acid structure of proline restricts it from forming hydrogen bonds with surrounding amino acids. Therefore, proline-rich sequences tend to be conformationally independent of proteins that they encounter and form relatively rigid extended structures (George and Heringa 2002). The two proline-rich linker peptides we selected to construct the trivalent STa–LTB–STb fusion were shown to act favorably to join the enterotoxins. First, the resulting fusion SLS protein elicited remarkably high antibody responses to both STa and STb in mice, suggesting that the fusion enhanced the immunogenicity of both STs. This indicates that, through the appropriate linkage by these two linkers, LTB effectively dedicates its immunologic determinants to STa and STb and does not disturb their normal conformation. As for the carrier portion, the majority of LTB’s immunogenicity was maintained because the titers of LTB-specific antibodies in the SLS-vaccinated group was over half of that in the LTB-vaccinated group. This suggests that the flanking of LTB with STa and STb only caused minor conformational changes in LTB.
It was encouraging that the SLS peptide was immunogenic for all the three component toxins as depicted by ELISA; more importantly, specific antibodies against SLS possessed the neutralizing activity to both heat-stable enterotoxin subtypes, STa and STb. This was an important consideration for SLS being used as an effective immunogen. With respect to the residual toxicity of SLS peptides, the toxicity of STa was dramatically diminished by a mutation at one disulfide-bridge-forming cysteine, but the toxicity of STb was still present for the full-length STb being constructed in the SLS fusion. Thus, further work is required to detoxify the STb constituent in SLS. Using the truncated STb may reduce its toxicity, but such an alternation may also render the molecule incapable of inducing neutralizing antibodies (Dubreuil et al. 1996). Considering the lack of impact of STb in adult animals, the SLS peptide still had the potential to be used to vaccinate pregnant dams for providing passive protection to their pups via the colostrum antibodies.
In practical application, since immunized dams are only needed to maintain a high level of immune responses during the suckling period for 3–4 weeks, the interval between the last immunization and challenge was chosen to be 2 weeks instead of a longer period. In the ETEC challenge experiments, SLS group showed the highest survival rate (70%) among the treated groups. LTB group had lower survival rate (40%) than SLS group, although mice in this group exhibited higher anti-LTB antibody titers. Somewhat unexpectedly, only 20% of mice in the F4ac group survived the challenge. The fact that the fully virulent ETEC F4ac strains used for the challenge produce all the three enterotoxins (i.e., LT, STa, and STb) may account for this outcome. In other words, the lack of anti-STs antibodies in mice from LTB or F4ac group may have contributed to their relatively low survival rates. These data suggest that specific antibodies to STa and STb in SLS-vaccinated mice were able to neutralize the STs’ toxicity in vivo and thus improve the resistance of mice to the lethal activity of ETEC infection. Other possible explanations for the inadequate protective immunity induced by killed E. coli F4ac bacteria are the degradation of surface antigens due to the multiple thawing–freezing cycles and the low expression level of F4ac fimbrial antigen of the E. coli strains cultured in CAYE medium which was originally designed to enhance enterotoxin production and is inferior to Minca medium for F4ac fimbriae production (Yokoyama et al. 1992). Additionally, no mice in the PBS group survived for more than 3 days after the challenge, showing that the challenge through intraperitoneal injection of ETEC F4ac was intense and likely far more severe than natural ETEC infection cases.
In conclusion, this work has described the construction of a trivalent E. coli enterotoxin STa–LTB–STb which is capable of eliciting extensive antitoxic antibody responses in mice and providing protective immunity against ETEC infection. Thus, this toxoid could be included in multivalent vaccines to provide broad-spectrum protection against diarrhea caused by ETEC expressing various fimbriae.
References
Allen KP, Randolph MM, Fleckenstein JM (2006) Importance of heat-labile enterotoxin in colonization of the adult mouse small intestine by human enterotoxigenic Escherichia coli strains. Infect Immun 74:869–875
Argos P (1990) An investigation of oligopeptides linking domains in protein tertiary structures and possible candidates for general gene fusion. J Mol Biol 211:943–958
Berberov EM, Zhou Y, Francis DH, Scott MA, Kachman SD, Moxey RA (2004) Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect Immun 72:3914–3924
Cardenas L, Clements JD (1993) Development of mucosal protection against the heat-stable enterotoxin (ST) of Escherichia coli by oral immunization with a genetic fusion delivered by a bacterial vector. Infect Immun 61:4629–4636
Chapman TA, Wu XY, Barchia I, Bettelheim KA, Driesen S, Trott D, Wilson M, Chin JJ (2006) Comparison of virulence gene profiles of Escherichia coli strains isolated from healthy and diarrheic swine. Appl Environ Microbiol 72:4782–4795
Clements JD (1990) Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect Immun 58:1159–1166
Crasto CJ, Feng JA (2000) LINKER: a program to generate linker sequences for fusion proteins. Protein Eng 13:309–312
Dubreuil JD (1997) Escherichia coli STb enterotoxin. Microbiology 143:1783–1795
Dubreuil JD (2008) Escherichia coli STb toxin and colibacillosis: knowing is half the battle. FEMS Microbiol Lett 278:137–145
Dubreuil JD, Letellier A, Harel J (1996) A recombinant Escherichia coli heat-stable enterotoxin b (STb) fusion protein eliciting neutralizing antibodies. FEMS Immunol Med Microbiol 13:317–323
Erume J, Berberov EM, Kachman SD, Scott MA, Zhou Y, Francis DH, Moxley RA (2008) Comparison of the contributions of heat-labile enterotoxin and heat-stable enterotoxin b to the virulence of enterotoxigenic Escherichia coli in F4ac receptor-positive young pigs. Infect Immun 76:3141–3149
Fujii Y, Hayashi M, Hitotsubashi S, Fuke Y, Yamanaka H, Okamoto K (1991) Purification and characterization of Escherichia coli heat-stable enterotoxin II. J Bacteriol 173:5516–5522
Gaastra W, Svennerholm AM (1996) Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol 4:444–452
George RA, Heringa J (2002) An analysis of protein domain linkers: their classification and role in protein folding. Protein Eng 15:871–879
Giannella RA (1976) Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun 14:95–99
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723
Jagusztyn-Krynicka EK, Clark-Curtiss JE, Curtiss R III (1993) Escherichia coli heat-labile toxin subunit B fusions with Streptococcus sobrinus antigens expressed by Salmonella typhimurium oral vaccine strains: importance of the linker for antigenicity and biological activities of the hybrid proteins. Infect Immun 61:1004–1015
Khan CM, Villarreal-Ramos B, Pierce RJ, Riveau G, Demarco de Hormaeche R, McNeill H, Ali T, Fairweather N, Chatfield S, Capron A (1994) Construction, expression, and immunogenicity of the Schistosoma mansoni P28 glutathione S-transferase as a genetic fusion to tetanus toxin fragment C in a live Aro attenuated vaccine strain of Salmonella. Proc Natl Acad Sci USA 91:11261–11265
Kupersztoch YM, Tachias K, Moomaw CR, Dreyfus LA, Urban R, Slaughter C, Whipp S (1990) Secretion of methanol-insoluble heat-stable enterotoxin (STB): energy- and secA-dependent conversion of pre-STB to an intermediate indistinguishable from the extracellular toxin. J Bacteriol 172:2427–2432
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227:680–685
Lawrence RM, Huang PT, Glick J, Oppenheim JD, Maas WK (1990) Expression of the cloned gene for enterotoxin STb of Escherichia coli. Infect Immun 58:970–977
Lowenadler B, Lake M, Elmblad A, Holmgren E, Holmgren J, Karlstrom A, Svennerholm AM (1991) A recombinant Escherichia coli heat-stable enterotoxin (STa) fusion protein eliciting anti-STa neutralizing antibodies. FEMS Microbiol Lett 66:271–277
Mundell DH, Anselmo CR, Wishnow RM (1976) Factors influencing heat-labile Escherichia coli enterotoxin activity. Infect Immun 14:383–388
Nagy B, Fekete PZ (2005) Enterotoxigenic Escherichia coli in veterinary medicine. Int J Med Microbiol 295:443–454
Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11:142–201
Qadri F, Svennerholm AM, Faruque AS, Sack RB (2005) Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18:465–483
Rosales-Mendoza S, Alpuche-Solis AG, Soria-Guerra RE, Moreno-Fierros L, Martinez-Gonzalez L, Herrera-Diaz A, Korban SS (2009) Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin, and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J 57:45–54
Rousset E, Harel J, Dubreuil JD (1998) Sulfatide from the pig jejunum brush border epithelial cell surface is involved in binding of Escherichia coli enterotoxin b. Infect Immun 66:5650–5658
Saarilahti HT, Palva ET, Holmgren J, Sanchez J (1989) Fusion of genes encoding Escherichia coli heat-stable enterotoxin and outer-membrane protein Ompc. Infect Immun 57:3663–3665
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Sanchez J, Svennerholm AM, Holmgren J (1988) Genetic fusion of a non-toxic heat-stable enterotoxin-related decapeptide antigen to cholera toxin B-subunit. FEBS Lett 241:110–114
Sizemore DR, Roland KL, Ryan US (2004) Enterotoxigenic Escherichia coli virulence factors and vaccine approaches. Expert Rev Vaccin 3:585–595
Staples SJ, Asher SE, Giannella RA (1980) Purification and characterization of heat-stable enterotoxin produced by a strain of E. coli pathogenic for man. J Biol Chem 10:4716–4721
Steinsland H, Valentiner-Branth P, Gjessing HK, Aaby P, Molbak K, Sommerfelt H (2003) Protection from natural infections with enterotoxigenic Escherichia coli: longitudinal study. Lancet 362:286–291
Svennerholm AM, Tobias J (2008) Vaccines against enterotoxigenic Escherichia coli. Expert Rev Vaccin 7:795–804
Tanaka T, Kuroda Y, Yokoyama S (2003) Characteristics and prediction of domain linker sequences in multi-domain proteins. J Struct Funct Genomics 4:79–85
Whipp SC (1990) Assay of enterotoxigenic Escherichia coli heat-stable toxin b in rats and mice. Infect Immun 58:930–934
Wolf MK (1997) Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev 10:569–584
Xue F, Gu Z, Feng JA (2004) LINKER: a web server to generate peptide sequences with extended conformation. Nucleic Acids Res 32:W562–W565
Yokoyama H, Peralta RC, Diaz R, Sendo S, Ikemori Z, Kodama Y (1992) Passive protective effect of chicken egg yolk immunoglobulins against experimental enterotoxigenic Escherichia coli infection in neonatal piglets. Infect Immun 60:998–1007
Zhang W, Berberov EM, Freeling J, He D, Moxley RA, Francis DH (2006) Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect Immun 74:3107–3114
Zhang WP, Zhang CX, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC (2010) Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect Immun 78:316–325
Acknowledgements
This work was conducted in part under the “MOE-AAFC PhD Research Program”, a collaboration of the Ministry of Education of China and Agriculture and Agri-Food Canada. We would like to acknowledge the peer review project program of Agri-Food Canada for partial support of this work. We are grateful to K. Munns for her suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, J., Xu, Y., He, M. et al. Protection of mice against enterotoxigenic E. coli by immunization with a polyvalent enterotoxin comprising a combination of LTB, STa, and STb. Appl Microbiol Biotechnol 89, 1885–1893 (2011). https://doi.org/10.1007/s00253-010-2991-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2991-7