Abstract
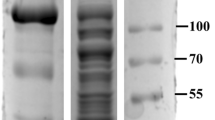
An efficient β-1,4-glucosidase (BGL) secreting strain, Agaricus arvensis, was isolated and identified. The relative molecular weight of the purified A. arvensis BGL was 98 kDa, as determined by sodium dodecylsulfate polyacrylamide gel electrophoresis, or 780 kDa by size exclusion chromatography, indicating that the enzyme is an octamer. Using a crude enzyme preparation, A. arvensis BGL was covalently immobilized onto functionalized silicon oxide nanoparticles with an immobilization efficiency of 158%. The apparent V max (k cat) values of free and immobilized BGL under standard assay conditions were 3,028 U mg protein−1 (4,945 s−1) and 3,347 U mg protein−1 (5,466 s−1), respectively. The immobilized BGL showed a higher optimum temperature and improved thermostability as compared to the free enzyme. The half-life at 65 °C showed a 288-fold improvement over the free BGL. After 25 cycles, the immobilized enzyme still retained 95% of the original activity, thus demonstrating its prospects for commercial applications. High specific activity, high immobilization efficiency, improved stability, and reusability of A. arvensis BGL make this enzyme of potential interest in a number of industrial applications.






Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cecchini DA, Serra I, Ubiali D, Terreni M, Albertini AM (2007) New active site oriented glyoxyl-agarose derivatives of Escherichia coli penicillin G acylase. BMC Biotechnol 7:54
Chauve M, Mathis H, Huc D, Casanave D, Monot F, Ferreira NL (2010) Comparative kinetic analysis of two fungal beta-glucosidases. Biotechnol Biofuels 3(1):3
Elyas KK, Mathew A, Sukumaran RK, Ali PP, Sapna K, Kumar SR, Mol KR (2010) Production optimization and properties of beta glucosidases from a marine fungus Aspergillus-SA 58. N Biotechnol. doi:10.1016/j.nbt.2010.02.007
Fessner WD, Anthonsen T (2009) Modern biocatalysis: stereoselective and environmentally friendly reactions. Wiley-VCH, Weinheim
Guisán JM, Penzol G, Armisen P, Bastida A, Blanco RM, Fernandez-Lafuente R, García-Junceda E (1996) Immobilization of enzymes acting on macromolecular substrates. Immobilization of enzymes and cells. Humana, Totowa, NJ, pp 261–275
Hanefeld U, Gardossi L, Magner E (2009) Understanding enzyme immobilisation. Chem Soc Rev 38(2):453–468
Itoh T, Ishii R, Matsuura S, Mizuguchi J, Hamakawa S, Hanaoka TA, Tsunoda T, Mizukami F (2010) Enhancement in thermal stability and resistance to denaturants of lipase encapsulated in mesoporous silica with alkyltrimethylammonium (CTAB). Colloids Surf B Biointerfaces 75(2):478–482
Joo AR, Jeya M, Lee KM, Sim WI, Kim JS, Kim IW, Kim YS, Oh DK, Gunasekaran P, Lee JK (2009) Purification and characterization of a beta-1,4-glucosidase from a newly isolated strain of Fomitopsis pinicola. Appl Microbiol Biotechnol 83(2):285–294
Kim J, Grate JW, Wang P (2006) Nanostructures for enzyme stabilization. Chem Eng Sci 61(3):1017–1026
Kim J, Grate JW, Wang P (2008) Nanobiocatalysis and its potential applications. Trends Biotechnol 26(11):639–646
Lee SM, Jin LH, Kim JH, Han SO, Na HB, Hyeon T, Koo YM, Kim J, Lee JH (2010) Beta-glucosidase coating on polymer nanofibers for improved cellulosic ethanol production. Bioprocess Biosyst Eng 33(1):141–147
Leite RSR, Gomes E, Da Silva R (2007) Characterization and comparison of thermostability of purified beta-glucosidases from a mesophilic Aureobasidium pullulans and a thermophilic Thermoascus aurantiacus. Proc Biochem 42(7):1101–1106
Libertino S, Giannazzo F, Aiello V, Scandurra A, Sinatra F, Renis M, Fichera M (2008) XPS and AFM characterization of the enzyme glucose oxidase immobilized on SiO(2) surfaces. Langmuir 24(5):1965–1972
Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS (2002) Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev 66(3):506–577
Mateo C, Abian O, Fernandez-Lafuente R, Guisan JM (2000) Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzyme Microb Technol 26(7):509–515
Polizzi KM, Bommarius AS, Broering JM, Chaparro-Riggers JF (2007) Stability of biocatalysts. Curr Opin Chem Biol 11(2):220–225
Rekuc A, Bryjak J, Szymanska K, Jarzebski AB (2010) Very stable silica-gel-bound laccase biocatalysts for the selective oxidation in continuous systems. Bioresour Technol 101(7):2076–2083
Riou C, Salmon JM, Vallier MJ, Gunata Z, Barre P (1998) Purification, characterization, and substrate specificity of a novel highly glucose-tolerant beta-glucosidase from Aspergillus oryzae. Appl Environ Microbiol 64(10):3607–3614
Tan YH, Liu M, Nolting B, Go JG, Gervay-Hague J, Liu GY (2008) A nanoengineering approach for investigation and regulation of protein immobilization. ACS Nano 2(11):2374–2384
Tiwari MK, Moon HJ, Jeya M, Lee JK (2010) Cloning and characterization of a thermostable xylitol dehydrogenase from Rhizobium etli CFN42. Appl Microbiol Biotechnol 87(2):571–581
Tu M, Zhang X, Kurabi A, Gilkes N, Mabee W, Saddler J (2006) Immobilization of beta-glucosidase on Eupergit C for lignocellulose hydrolysis. Biotechnol Lett 28(3):151–156
Wang X, Zhou D, Sinniah K, Clarke C, Birch L, Li H, Rayment T, Abell C (2006) Electrostatic orientation of enzymes on surfaces for ligand screening probed by force spectroscopy. Langmuir 22(3):887–892
Wang P, Hu X, Cook S, Hwang H-M (2009) Influence of silica-derived nano-supporters on cellobiase after immobilization. Appl Biochem Biotechnol 158(1):88–96
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Wong LS, Khan F, Micklefield J (2009) Selective covalent protein immobilization: strategies and applications. Chem Rev 109(9):4025–4053
Zhang YW, Prabhu P, Lee JK (2009) Immobilization of Bacillus licheniformis l-arabinose isomerase for semi-continuous l-ribulose production. Biosci Biotechnol Biochem 73(10):2234–2239
Acknowledgment
This study was supported by a grant (code 2008A0080126) from ARPC, Republic of Korea. This work was also supported by a grant (code 20070301034024) from Biogreen 21 Program, Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Singh, R.K., Zhang, YW., Nguyen, NPT. et al. Covalent immobilization of β-1,4-glucosidase from Agaricus arvensis onto functionalized silicon oxide nanoparticles. Appl Microbiol Biotechnol 89, 337–344 (2011). https://doi.org/10.1007/s00253-010-2768-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2768-z